Effect of Intensive Blood-Pressure Treatment on Patient-Reported Outcomes
- PMID: 28834483
- PMCID: PMC5706112
- DOI: 10.1056/NEJMoa1611179
Effect of Intensive Blood-Pressure Treatment on Patient-Reported Outcomes
Abstract
Background: The previously published results of the Systolic Blood Pressure Intervention Trial showed that among participants with hypertension and an increased cardiovascular risk, but without diabetes, the rates of cardiovascular events were lower among those who were assigned to a target systolic blood pressure of less than 120 mm Hg (intensive treatment) than among those who were assigned to a target of less than 140 mm Hg (standard treatment). Whether such intensive treatment affected patient-reported outcomes was uncertain; those results from the trial are reported here.
Methods: We randomly assigned 9361 participants with hypertension to a systolic blood-pressure target of less than 120 mm Hg or a target of less than 140 mm Hg. Patient-reported outcome measures included the scores on the Physical Component Summary (PCS) and Mental Component Summary (MCS) of the Veterans RAND 12-Item Health Survey, the Patient Health Questionnaire 9-item depression scale (PHQ-9), patient-reported satisfaction with their blood-pressure care and blood-pressure medications, and adherence to blood-pressure medications. We compared the scores in the intensive-treatment group with those in the standard-treatment group among all participants and among participants stratified according to physical and cognitive function.
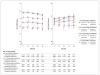
Results: Participants who received intensive treatment received an average of one additional antihypertensive medication, and the systolic blood pressure was 14.8 mm Hg (95% confidence interval, 14.3 to 15.4) lower in the group that received intensive treatment than in the group that received standard treatment. Mean PCS, MCS, and PHQ-9 scores were relatively stable over a median of 3 years of follow-up, with no significant differences between the two treatment groups. No significant differences between the treatment groups were noted when participants were stratified according to baseline measures of physical or cognitive function. Satisfaction with blood-pressure care was high in both treatment groups, and we found no significant difference in adherence to blood-pressure medications.
Conclusions: Patient-reported outcomes among participants who received intensive treatment, which targeted a systolic blood pressure of less than 120 mm Hg, were similar to those among participants who received standard treatment, including among participants with decreased physical or cognitive function. (Funded by the National Institutes of Health; SPRINT ClinicalTrials.gov number, NCT01206062 .).
Figures


Comment in
-
Intensive Blood-Pressure Treatment and Patient-Reported Outcomes.N Engl J Med. 2017 Nov 23;377(21):2096-7. doi: 10.1056/NEJMc1712573. N Engl J Med. 2017. PMID: 29182238 No abstract available.
-
More From SPRINT (Systolic Blood Pressure Intervention Trial): A Closer Look at the Price of Intensive Blood Pressure Control.Am J Kidney Dis. 2018 May;71(5):611-614. doi: 10.1053/j.ajkd.2017.11.008. Am J Kidney Dis. 2018. PMID: 29352606 No abstract available.
References
-
- Ortiz E, James PA. Let’s not SPRINT to judgment about new blood pressure goals. Ann Intern Med. 2016;165:889–90. - PubMed
-
- Saper CB. How low can you go? Ann Neurol. 2015;78:665–6. - PubMed
-
- Schiffrin EL, Calhoun DA, Flack JM. SPRINT proves that lower is better for nondiabetic high-risk patients, but at a price. Am J Hypertens. 2016;29:2–4. - PubMed
-
- Moonen JEF, Foster-Dingley JC, de Ruijter W, et al. Effect of discontinuation of antihypertensive treatment in elderly people on cognitive functioning — the DANTE Study Leiden. JAMA Intern Med. 2015;175:1622–30. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UL1 TR000433/TR/NCATS NIH HHS/United States
- UL1 TR000445/TR/NCATS NIH HHS/United States
- UL1 TR000064/TR/NCATS NIH HHS/United States
- UL1 TR000075/TR/NCATS NIH HHS/United States
- P30 DK079626/DK/NIDDK NIH HHS/United States
- UL1 TR000093/TR/NCATS NIH HHS/United States
- UL1 RR025755/RR/NCRR NIH HHS/United States
- UL1 RR024134/RR/NCRR NIH HHS/United States
- HHSN268200900048C/HL/NHLBI NIH HHS/United States
- UL1 TR000005/TR/NCATS NIH HHS/United States
- HHSN268200900040C/HL/NHLBI NIH HHS/United States
- HHSN268200900046C/HL/NHLBI NIH HHS/United States
- P30 GM103337/GM/NIGMS NIH HHS/United States
- UL1 TR001064/TR/NCATS NIH HHS/United States
- UL1 RR025752/RR/NCRR NIH HHS/United States
- UL1 RR025771/RR/NCRR NIH HHS/United States
- HHSN268200900049C/HL/NHLBI NIH HHS/United States
- HHSN268200900047C/HL/NHLBI NIH HHS/United States
- UL1 TR001427/TR/NCATS NIH HHS/United States
- UL1 TR000003/TR/NCATS NIH HHS/United States
- U54 TR0000017 UNIVERSITY OF TEXAS SOUTHWESTERN/TR/NCATS NIH HHS/United States
- UL1 TR000050/TR/NCATS NIH HHS/United States
- UL1 TR000439/TR/NCATS NIH HHS/United States
- UL1 TR000073/TR/NCATS NIH HHS/United States
- UL1 TR000002/TR/NCATS NIH HHS/United States
- UL1 TR002240/TR/NCATS NIH HHS/United States
- UL1 TR000105/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
