Zscan4 Inhibits Maintenance DNA Methylation to Facilitate Telomere Elongation in Mouse Embryonic Stem Cells
- PMID: 28834755
- PMCID: PMC5595351
- DOI: 10.1016/j.celrep.2017.07.070
Zscan4 Inhibits Maintenance DNA Methylation to Facilitate Telomere Elongation in Mouse Embryonic Stem Cells
Abstract
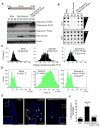
Proper telomere length is essential for embryonic stem cell (ESC) self-renewal and pluripotency. Mouse ESCs (mESCs) sporadically convert to a transient totipotent state similar to that of two-cell (2C) embryos to recover shortened telomeres. Zscan4, which exhibits a burst of expression in 2C-like mESCs, is required for telomere extension in these cells. However, the mechanism by which Zscan4 extends telomeres remains elusive. Here, we show that Zscan4 facilitates telomere elongation by inducing global DNA demethylation through downregulation of Uhrf1 and Dnmt1, major components of the maintenance DNA methylation machinery. Mechanistically, Zscan4 recruits Uhrf1 and Dnmt1 and promotes their degradation, which depends on the E3 ubiquitin ligase activity of Uhrf1. Blocking DNA demethylation prevents telomere elongation associated with Zscan4 expression, suggesting that DNA demethylation mediates the effect of Zscan4. Our results define a molecular pathway that contributes to the maintenance of telomere length homeostasis in mESCs.
Keywords: DNA methylation; Dnmt1; ESCs; Uhrf1; Zscan4; telomere.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures






References
-
- Benetti R, Gonzalo S, Jaco I, Munoz P, Gonzalez S, Schoeftner S, Murchison E, Andl T, Chen T, Klatt P, et al. A mammalian microRNA cluster controls DNA methylation and telomere recombination via Rbl2-dependent regulation of DNA methyltransferases. Nat Struct Mol Biol. 2008;15:268–279. - PMC - PubMed
-
- Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet. 2005;6:611–622. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

