A parallel-group randomized clinical trial of individually tailored, multidisciplinary, palliative rehabilitation for patients with newly diagnosed advanced cancer: the Pal-Rehab study protocol
- PMID: 28835218
- PMCID: PMC5569500
- DOI: 10.1186/s12885-017-3558-0
A parallel-group randomized clinical trial of individually tailored, multidisciplinary, palliative rehabilitation for patients with newly diagnosed advanced cancer: the Pal-Rehab study protocol
Abstract
Background: The effect of early palliative care and rehabilitation on the quality of life of patients with advanced cancer has been only sparsely described and needs further investigation. In the present trial we combine elements of early, specialized palliative care with cancer rehabilitation in a 12-week individually tailored, palliative rehabilitation program initiated shortly after a diagnosis of advanced cancer.
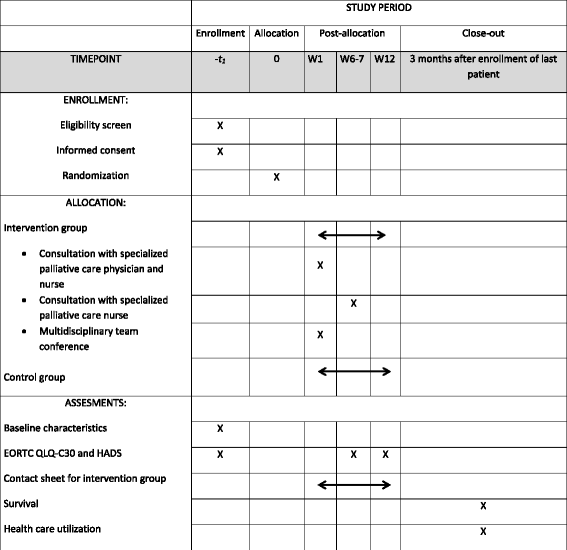
Methods: This single center, randomized, controlled trial will include 300 patients with newly diagnosed advanced cancer recruited from the Department of Oncology, Vejle Hospital. The patients are randomized to a specialized palliative rehabilitation intervention integrated in standard oncology care or to standard oncology care alone. The intervention consists of a multidisciplinary group program, individual consultations, or a combination of both. At baseline and after six and 12 weeks the patients will be asked to fill out questionnaires on symptoms, quality of life, and symptoms of depression and anxiety. Among the symptoms and problems assessed, patients are asked to indicate the problem they need help with to the largest extent. The effect of the intervention on this problem is the primary outcome measure of the study. Secondary outcome measures include survival and economic consequences.
Discussion: To our knowledge the Pal-Rehab study is the first randomized, controlled, phase III trial to evaluate individually tailored, palliative rehabilitation in standard oncology care initiated shortly after an advanced cancer diagnosis. The study will contribute with evidence on the effectiveness of implementing early palliative care in standard oncology treatment and hopefully offer new knowledge and future directions as to the content of palliative rehabilitation programs.
Trial registration: Clinicaltrials.gov Identifier: NCT02332317 , registered retrospectively on December 30, 2014. One study participant had been enrolled at the time.
Keywords: Advanced cancer; Cost-effectiveness; Early integrated care; Palliative care; Patient involvement; Quality of life research; Randomized clinical trial; Rehabilitation; Study protocol; Supportive care.
Conflict of interest statement
Authors’ information
Authors LN and LHJ are affiliated with OPEN, Odense Patient data Explorative Network, Odense University Hospital, Odense, Denmark.
Tove Bahn Vejlgaard is former Head of the PalliativeTeam, Vejle (retired).
Ethics approval and consent to participate
All study participants are enrolled following verbal and written informed consent. The study protocol including the written material intended for potential study participants and the informed content sheet was approved by The Regional Committees on Health Research Ethics for Southern Denmark on April 2nd, 2014 (Project ID S-20140038).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
-
- World Health Organization | WHO Definition of Palliative Care. http://www.who.int/cancer/palliative/definition/en/ (2012). Accessed 23 Aug 2016.
-
- Danish Palliative Database Annual Report (2015). http://www.dmcgpal.dk/573/%C3%85rsrapport-fra-dansk-palliativ-database
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical