Does dapagliflozin regress left ventricular hypertrophy in patients with type 2 diabetes? A prospective, double-blind, randomised, placebo-controlled study
- PMID: 28835229
- PMCID: PMC5569551
- DOI: 10.1186/s12872-017-0663-6
Does dapagliflozin regress left ventricular hypertrophy in patients with type 2 diabetes? A prospective, double-blind, randomised, placebo-controlled study
Abstract
Background: Patients with diabetes have a two to fourfold increased risk for development of and death from cardiovascular disease [CVD]. The current oral hypoglycaemic agents result in limited reduction in this cardiovascular risk. Sodium glucose linked co-transporter type 2 [SGLT2] inhibitors are a relatively new class of antidiabetic agent that have been shown to have potential cardiovascular benefits. In support of this, the EMPA-REG trial showed a striking 38% and 35% reduction in cardiovascular mortality and heart failure [HF] hospitalisation respectively. The exact mechanism (s) responsible for these effects remain (s) unclear. One potential mechanism is regression of Left ventricular hypertrophy (LVH).
Methods: The DAPA-LVH trial is a prospective, double-blind, randomised, placebo-controlled 'proof of concept' single-centre study that has been ongoing since January 2017. It is designed specifically to assess whether the SGLT2 inhibitor dapagliflozin regresses left ventricular [LV] mass in patients with diabetes and left ventricular hypertrophy [LVH]. We are utilising cardiac and abdominal magnetic resonance imaging [MRI] and ambulatory blood pressure monitoring to quantify the cardiovascular and systemic effects of dapagliflozin 10 mg once daily against standard care over a 1 year observation period. The primary endpoint is to detect the changes in LV mass. The secondary outcomes are to assess the changes in, LV volumes, blood pressure, weight, visceral and subcutaneous fat.
Discussion: This trial will be able to determine if SGLT2 inhibitor therapy reduces LV mass in patient with diabetes and LVH thereby strengthening their position as oral hypoglycaemic agents with cardioprotective benefits.
Trial registration: Clinical Trials.gov: NCT02956811 . Registered November 2016.
Keywords: Cardiac MRI; Diabetes; Left ventricular hypertrophy; Mechanistic trial; SGLT2 inhibitor.
Conflict of interest statement
Ethics approval and consent to participate
The study has been granted ethics approval by the East of Scotland Research Ethics Service [16/ES/0131].
Consent for publication
This article does not contain any individual person’s data thus consent for publication is not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
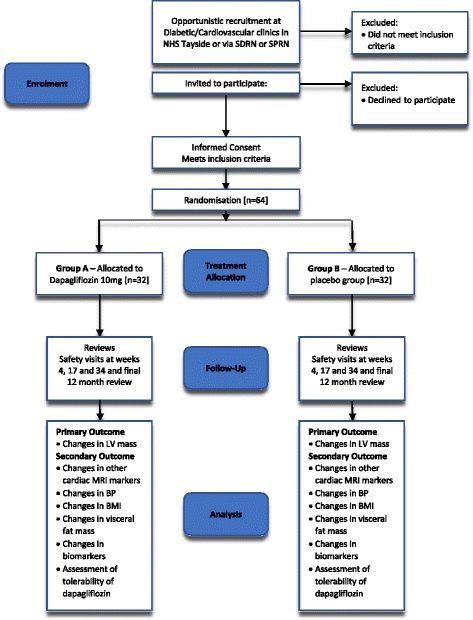
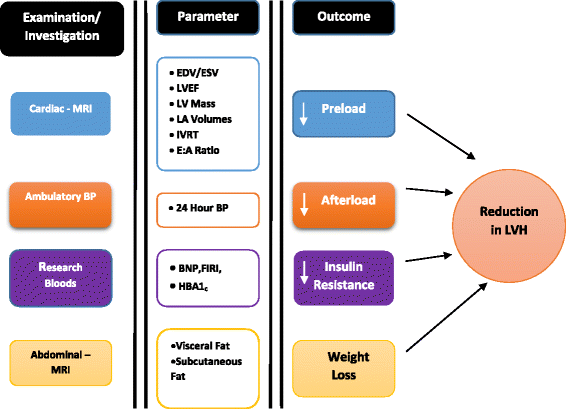
References
-
- Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes [UKPDS 35]: prospective observational study. BMJ Clinical Research Ed. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

