Conservation and divergence of small RNA pathways and microRNAs in land plants
- PMID: 28835265
- PMCID: PMC5569507
- DOI: 10.1186/s13059-017-1291-2
Conservation and divergence of small RNA pathways and microRNAs in land plants
Abstract
Background: As key regulators of gene expression in eukaryotes, small RNAs have been characterized in many seed plants, and pathways for their biogenesis, degradation, and action have been defined in model angiosperms. However, both small RNAs themselves and small RNA pathways are not well characterized in other land plants such as lycophytes and ferns, preventing a comprehensive evolutionary perspective on small RNAs in land plants.
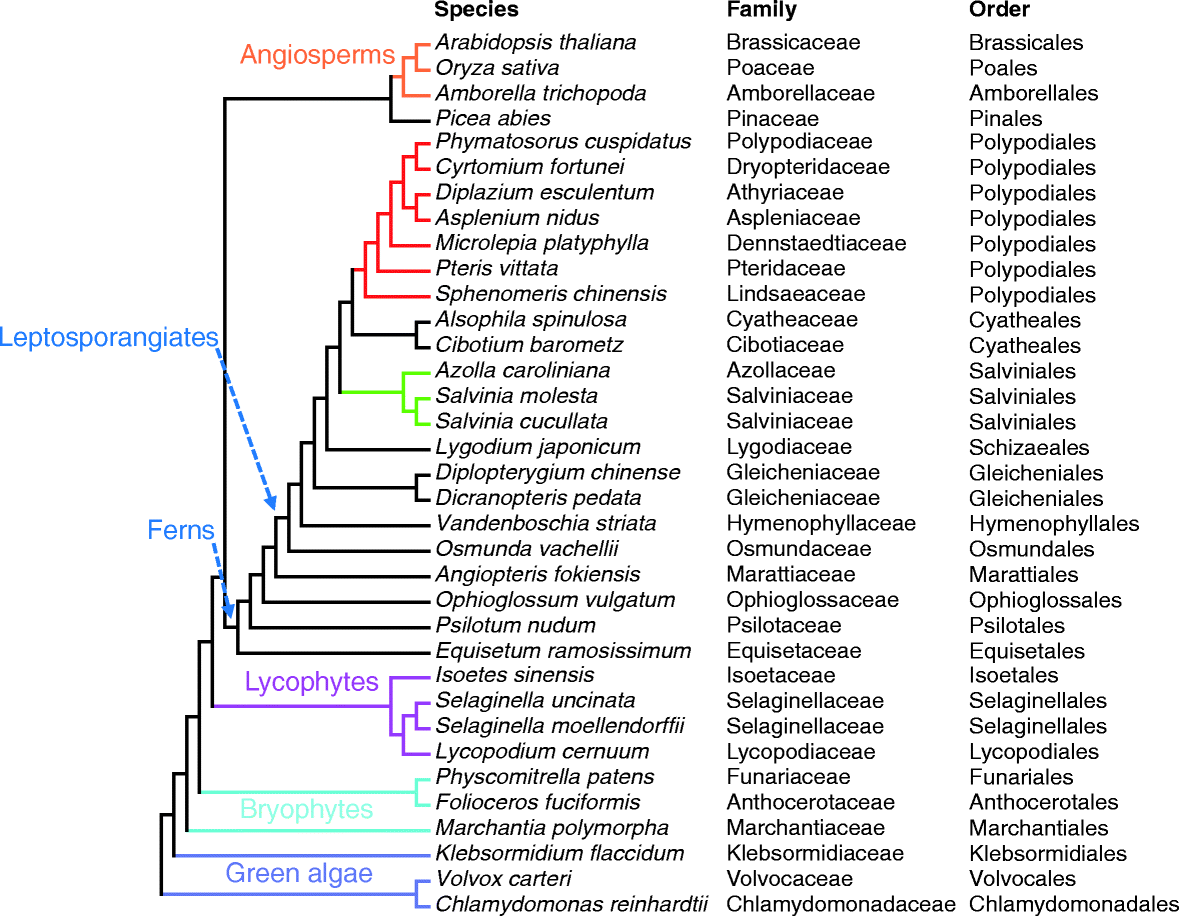
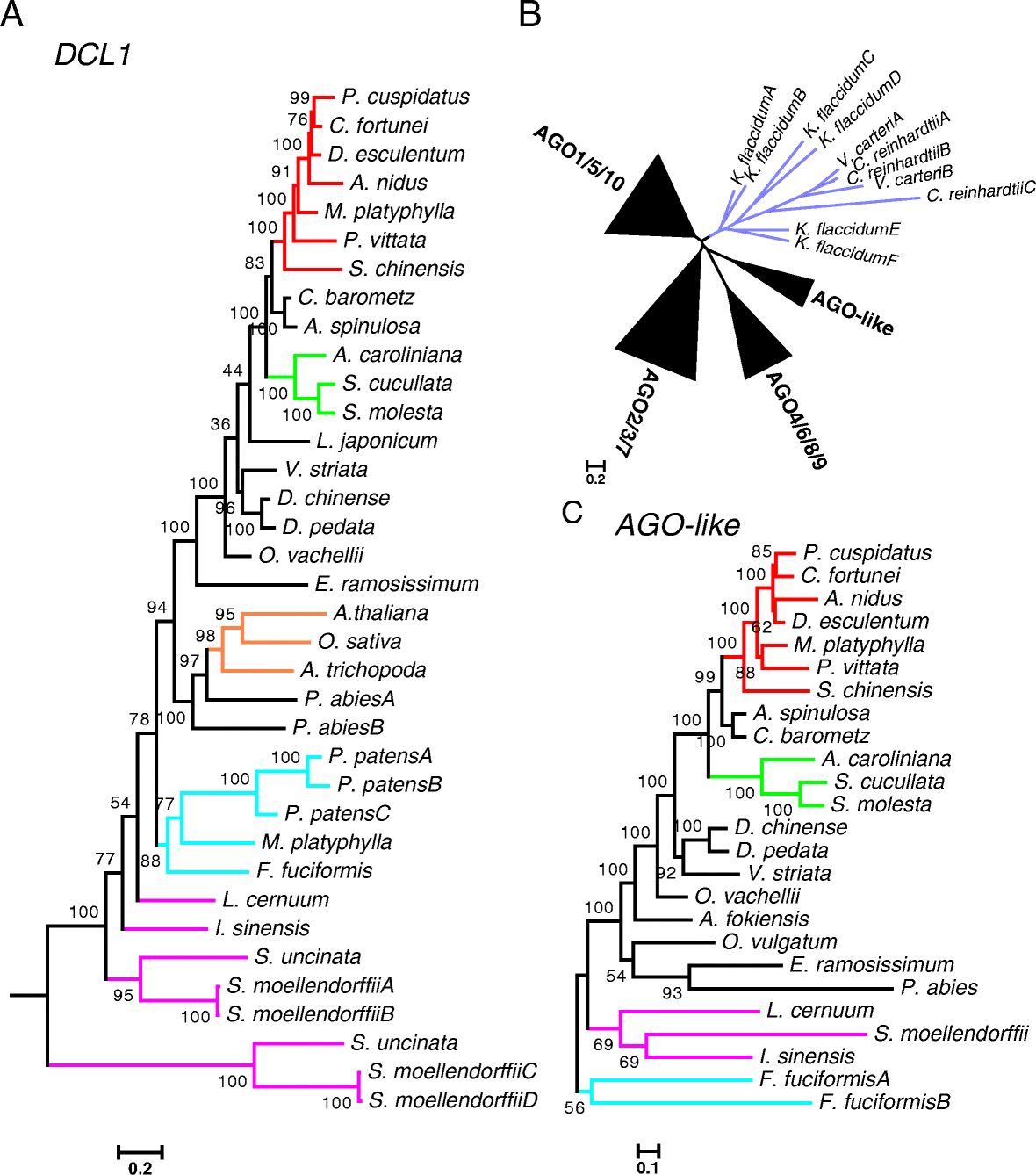
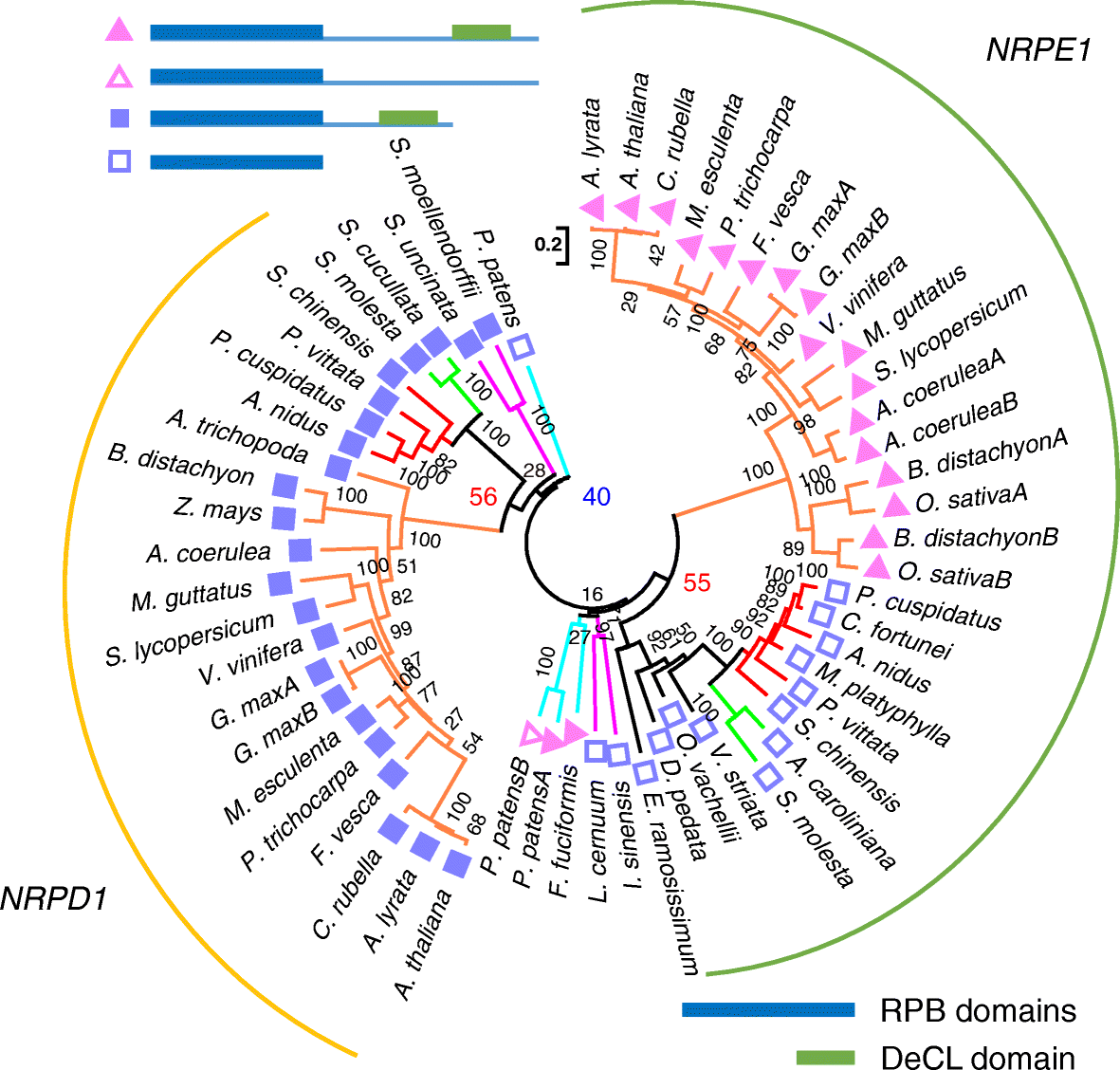
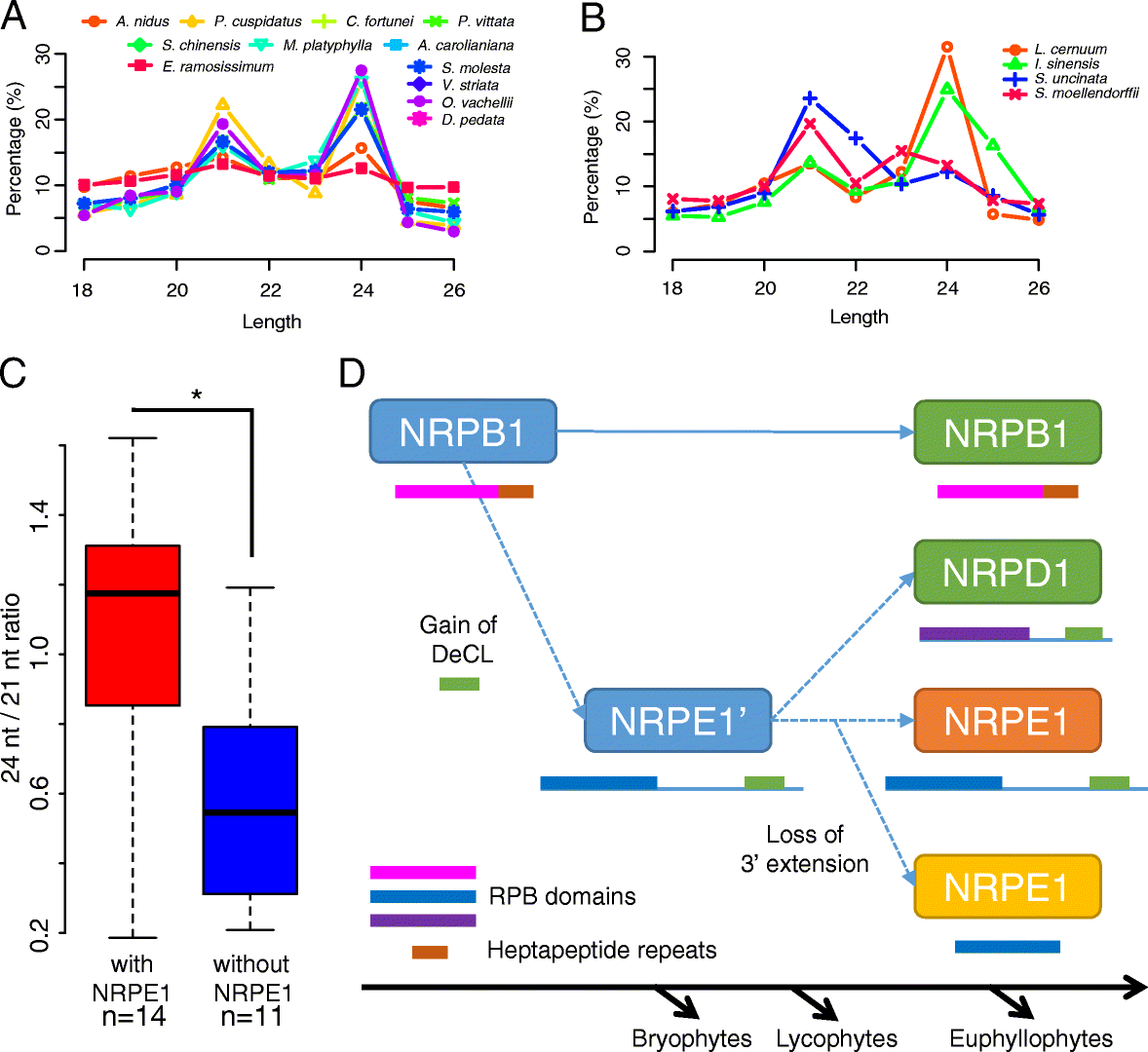
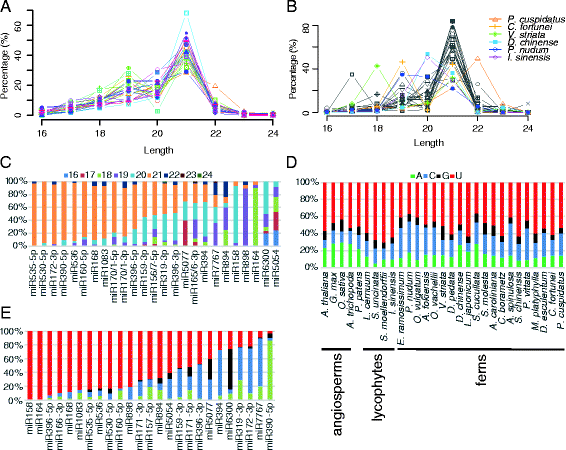
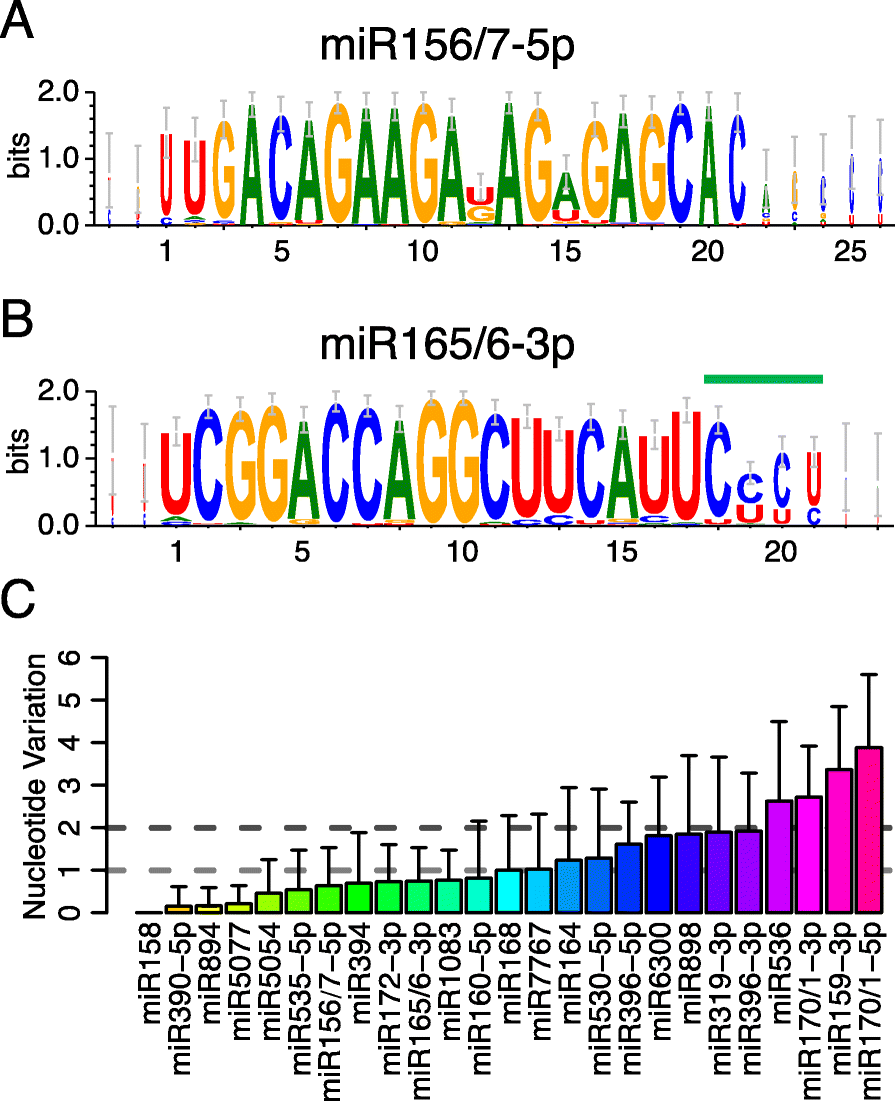
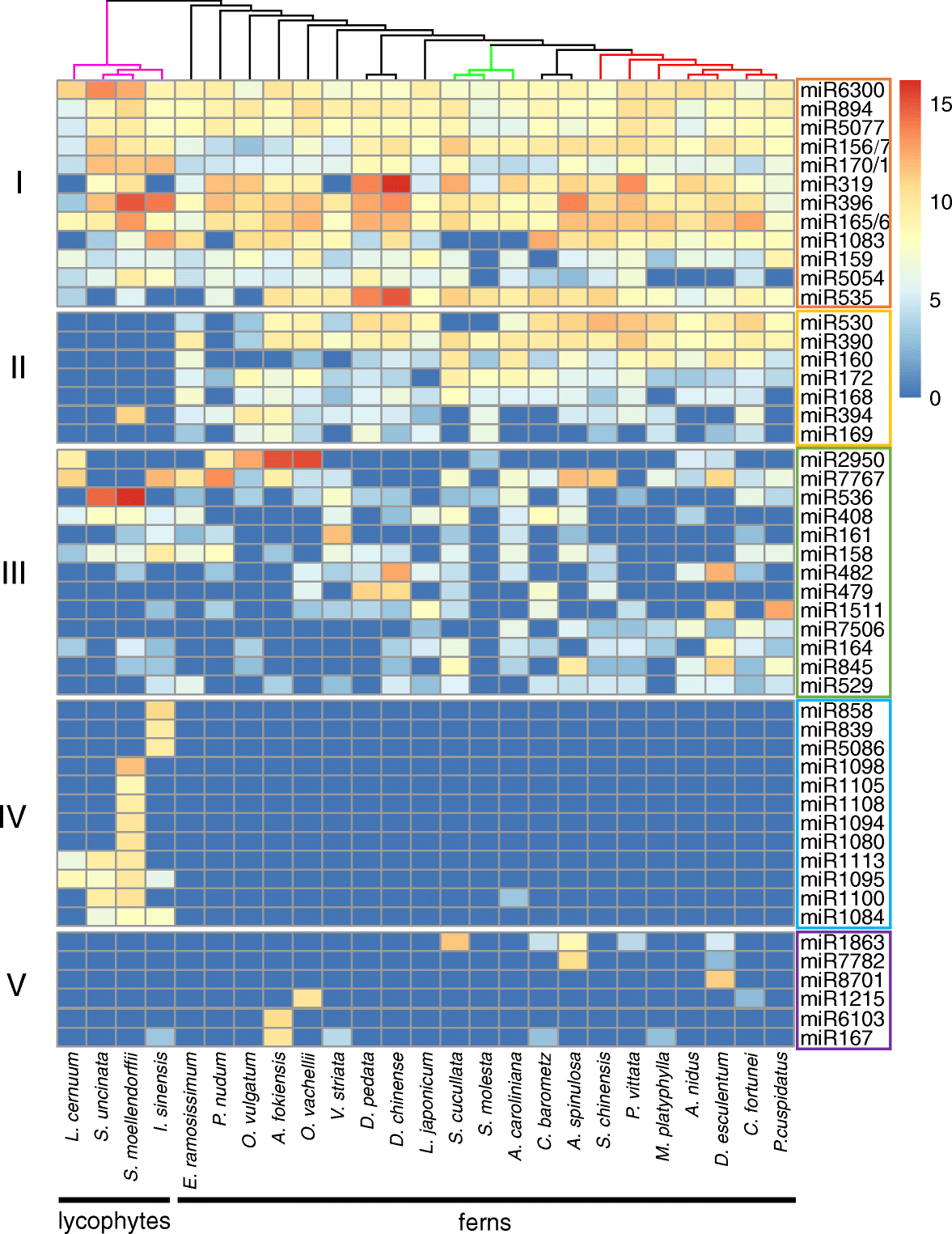
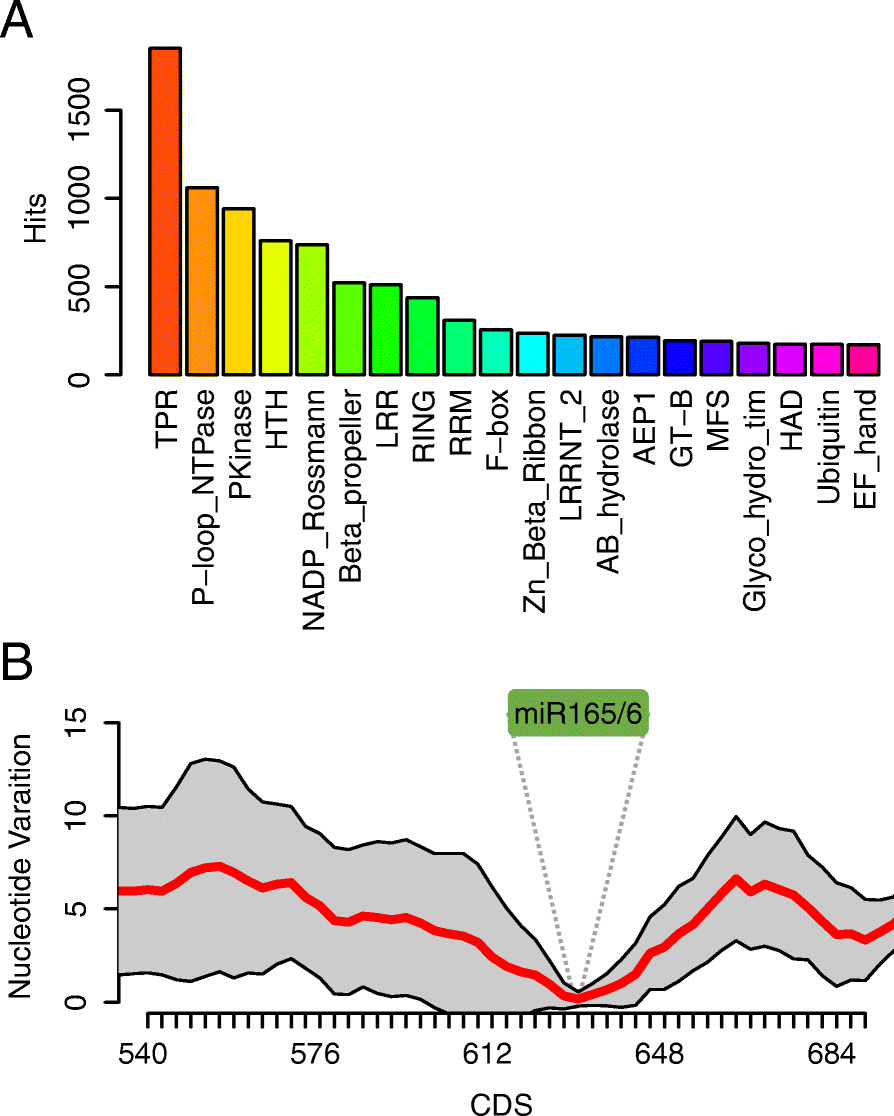
Results: Using 25 representatives from major lineages of lycophytes and ferns, most of which lack sequenced genomes, we characterized small RNAs and small RNA pathways in these plants. We identified homologs of DICER-LIKE (DCL), ARGONAUTE (AGO), and other genes involved in small RNA pathways, predicted over 2600 conserved microRNA (miRNA) candidates, and performed phylogenetic analyses on small RNA pathways as well as miRNAs. Pathways underlying miRNA biogenesis, degradation, and activity were established in the common ancestor of land plants, but the 24-nucleotide siRNA pathway that guides DNA methylation is incomplete in sister species of seed plants, especially lycophytes. We show that the functional diversification of key gene families such as DCL and AGO as observed in angiosperms occurred early in land plants followed by parallel expansion of the AGO family in ferns and angiosperms. We uncovered a conserved AGO subfamily absent in angiosperms.
Conclusions: Our phylogenetic analyses of miRNAs in bryophytes, lycophytes, ferns, and angiosperms refine the time-of-origin for conserved miRNA families as well as small RNA machinery in land plants.
Keywords: Argonaute; DICER-LIKE; Evolution; Fern; Lycophyte; RdDM; Small RNA; miRNA.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

