Anti-inflammatory and immunomodulatory mechanisms of atorvastatin in a murine model of traumatic brain injury
- PMID: 28835272
- PMCID: PMC5569493
- DOI: 10.1186/s12974-017-0934-2
Anti-inflammatory and immunomodulatory mechanisms of atorvastatin in a murine model of traumatic brain injury
Abstract
Background: Neuroinflammation is an important secondary injury mechanism that has dual beneficial and detrimental roles in the pathophysiology of traumatic brain injury (TBI). Compelling data indicate that statins, a group of lipid-lowering drugs, also have extensive immunomodulatory and anti-inflammatory properties. Among statins, atorvastatin has been demonstrated as a neuroprotective agent in experimental TBI; however, there is a lack of evidence regarding its effects on neuroinflammation during the acute phase of TBI. The current study aimed to evaluate the effects of atorvastatin therapy on modulating the immune reaction, and to explore the possible involvement of peripheral leukocyte invasion and microglia/macrophage polarization in the acute period post-TBI.
Methods: C57BL/6 mice were subjected to TBI using a controlled cortical impact (CCI) device. Either atorvastatin or vehicle saline was administered orally starting 1 h post-TBI for three consecutive days. Short-term neurological deficits were evaluated using the modified neurological severity score (mNSS) and Rota-rod. Brain-invading leukocyte subpopulations were analyzed by flow cytometry and immunohistochemistry. Pro- and anti-inflammatory cytokines and chemokines were examined using enzyme-linked immunosorbent assay (ELISA). Markers of classically activated (M1) and alternatively activated (M2) microglia/macrophages were then determined by quantitative real-time PCR (qRT-PCR) and flow cytometry. Neuronal apoptosis was identified by double staining of terminal deoxynucleotidyl transferase-dUTP nick end labeling (TUNEL) staining and immunofluorescence labeling for neuronal nuclei (NeuN).
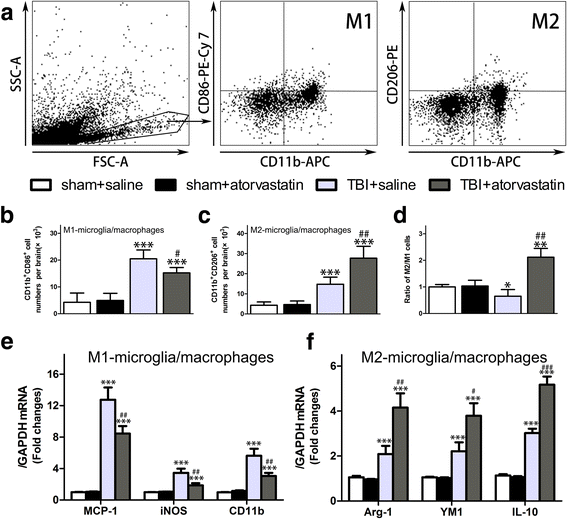
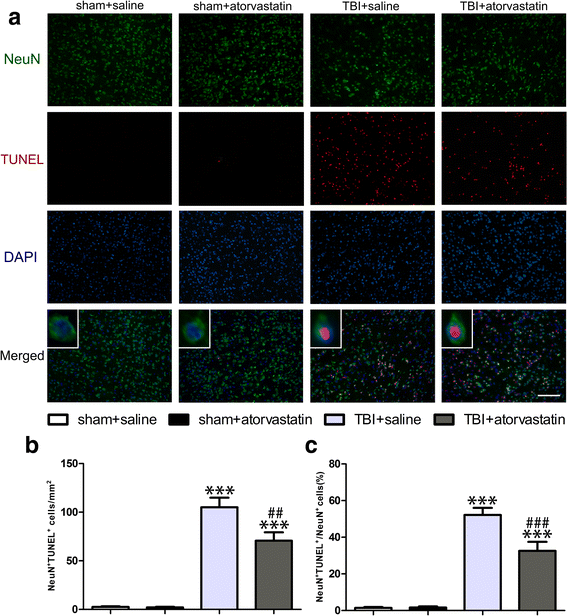
Results: Acute treatment with atorvastatin at doses of 1 mg/kg/day significantly reduced neuronal apoptosis and improved behavioral deficits. Invasions of T cells, neutrophils and natural killer (NK) cells were attenuated profoundly after atorvastatin therapy, as was the production of pro-inflammatory cytokines (IFN-γ and IL-6) and chemokines (RANTES and IP-10). Notably, atorvastatin treatment significantly increased the proportion of regulatory T cells (Tregs) in both the peripheral spleen and brain, and at the same time, increased their main effector cytokines IL-10 and TGF-β1. We also found that atorvastatin significantly attenuated total microglia/macrophage activation but augmented the M2/M1 ratio by both inhibiting M1 polarization and enhancing M2 polarization.
Conclusions: Our data demonstrated that acute atorvastatin administration could modulate post-TBI neuroinflammation effectively, via a mechanism that involves altering peripheral leukocyte invasion and the alternative polarization of microglia/macrophages.
Keywords: Anti-inflammation; Atorvastatin; Immunomodulation; Leukocyte; Microglia/macrophage subtype; Traumatic brain injury.
Conflict of interest statement
Ethics approval
All experimental procedures used in this study were approved by the Animal Ethics Committee of the Tianjin Medical University (Tianjin, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

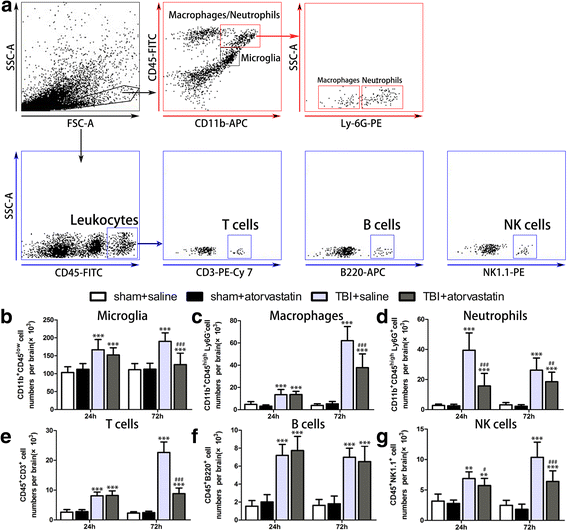
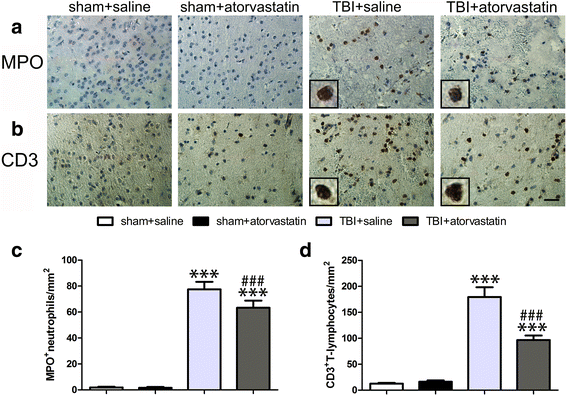

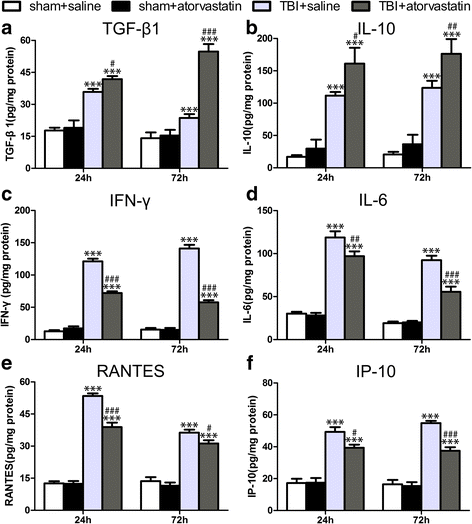
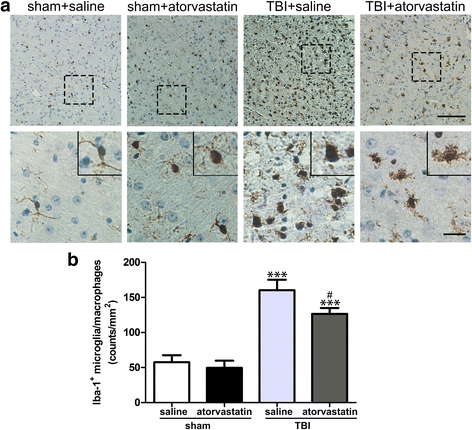
References
-
- Mishra SK, Kumar BS, Khushu S, Singh AK, Gangenahalli G. Early monitoring and quantitative evaluation of macrophage infiltration after experimental traumatic brain injury: a magnetic resonance imaging and flow cytometric analysis. Mol Cell Neurosci. 2017;78:25–34. doi: 10.1016/j.mcn.2016.11.008. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

