Alemtuzumab CARE-MS I 5-year follow-up: Durable efficacy in the absence of continuous MS therapy
- PMID: 28835401
- PMCID: PMC5595278
- DOI: 10.1212/WNL.0000000000004313
Alemtuzumab CARE-MS I 5-year follow-up: Durable efficacy in the absence of continuous MS therapy
Erratum in
-
Alemtuzumab CARE-MS I 5-year follow-up: Durable efficacy in the absence of continuous MS therapy.Neurology. 2018 Apr 17;90(16):755. doi: 10.1212/WNL.0000000000004908. Neurology. 2018. PMID: 29661899 Free PMC article. No abstract available.
Abstract
Objective: To evaluate 5-year efficacy and safety of alemtuzumab in treatment-naive patients with active relapsing-remitting MS (RRMS) (CARE-MS I; NCT00530348).
Methods: Alemtuzumab-treated patients received treatment courses at baseline and 12 months later; after the core study, they could enter an extension (NCT00930553) with as-needed alemtuzumab retreatment for relapse or MRI activity. Assessments included annualized relapse rate (ARR), 6-month confirmed disability worsening (CDW; ≥1-point Expanded Disability Status Scale [EDSS] score increase [≥1.5 if baseline EDSS = 0]), 6-month confirmed disability improvement (CDI; ≥1-point EDSS decrease [baseline score ≥2.0]), no evidence of disease activity (NEDA), brain volume loss (BVL), and adverse events (AEs).
Results: Most alemtuzumab-treated patients (95.1%) completing CARE-MS I enrolled in the extension; 68.5% received no additional alemtuzumab treatment. ARR remained low in years 3, 4, and 5 (0.19, 0.14, and 0.15). Over years 0-5, 79.7% were free of 6-month CDW; 33.4% achieved 6-month CDI. Most patients (61.7%, 60.2%, and 62.4%) had NEDA in years 3, 4, and 5. Median yearly BVL improved over years 2-4, remaining low in year 5 (years 1-5: -0.59%, -0.25%, -0.19%, -0.15%, and -0.20%). Exposure-adjusted incidence rates of most AEs declined in the extension relative to the core study. Thyroid disorder incidences peaked at year 3 and subsequently declined.
Conclusions: Based on these data, alemtuzumab provides durable efficacy through 5 years in the absence of continuous treatment, with most patients not receiving additional courses.
Clinicaltrialsgov identifier: NCT00530348; NCT00930553.
Classification of evidence: This study provides Class III evidence that alemtuzumab durably improves efficacy outcomes and slows BVL in patients with RRMS.
Copyright © 2017 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures
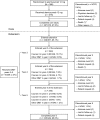


References
-
- Weber MS, Hemmer B. Cooperation of B cells and T cells in the pathogenesis of multiple sclerosis. Results Probl Cell Differ 2010;51:115–126. - PubMed
-
- Cox AL, Thompson SA, Jones JL, et al. Lymphocyte homeostasis following therapeutic lymphocyte depletion in multiple sclerosis. Eur J Immunol 2005;35:3332–3342. - PubMed
-
- CAMMS223 Trial Investigators, Coles AJ, Compston DA, et al. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med 2008;359:1786–1801. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
