FDA Approval Summary: Pembrolizumab for Treatment of Metastatic Non-Small Cell Lung Cancer: First-Line Therapy and Beyond
- PMID: 28835513
- PMCID: PMC5679831
- DOI: 10.1634/theoncologist.2017-0078
FDA Approval Summary: Pembrolizumab for Treatment of Metastatic Non-Small Cell Lung Cancer: First-Line Therapy and Beyond
Abstract
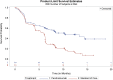
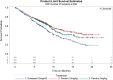
On October 24, 2016, the U.S. Food and Drug Administration (FDA) approved pembrolizumab (Keytruda; Merck & Co., Inc., https://www.merck.com) for treatment of patients with metastatic non-small cell lung cancer (mNSCLC) whose tumors express programmed death-ligand 1 (PD-L1) as determined by an FDA-approved test, as follows: (a) first-line treatment of patients with mNSCLC whose tumors have high PD-L1 expression (tumor proportion score [TPS] ≥50%), with no epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) genomic tumor aberrations, and (b) treatment of patients with mNSCLC whose tumors express PD-L1 (TPS ≥1%), with disease progression on or after platinum-containing chemotherapy. Patients with EGFR or ALK genomic tumor aberrations should have disease progression on FDA-approved therapy for these aberrations prior to receiving pembrolizumab.Approval was based on two randomized, open-label, active-controlled trials demonstrating statistically significant improvements in progression-free survival (PFS) and overall survival (OS) for patients randomized to pembrolizumab compared with chemotherapy. In KEYNOTE-024, patients with previously untreated mNSCLC who received pembrolizumab (200 mg intravenously [IV] every 3 weeks) had a statistically significant improvement in OS (hazard ratio [HR] 0.60; 95% confidence interval [CI]: 0.41-0.89; p = .005), and significant improvement in PFS (HR 0.50; 95% CI: 0.37-0.68; p < .001). In KEYNOTE-010, patients with disease progression on or after platinum-containing chemotherapy received pembrolizumab IV 2 mg/kg, 10 mg/kg, or docetaxel 75 mg/m2 every 3 weeks. The HR and p value for OS was 0.71 (95% CI: 0.58-0.88), p < .001 comparing pembrolizumab 2 mg/kg with chemotherapy and the HR and p value for OS was 0.61 (95% CI: 0.49-0.75), p < .001 comparing pembrolizumab 10 mg/kg with chemotherapy.
Implications for practice: This is the first U.S. Food and Drug Administration approval of a checkpoint inhibitor for first-line treatment of lung cancer. This approval expands the pembrolizumab indication in second-line treatment of lung cancer to include all patients with programmed death-ligand 1-expressing non-small cell lung cancer.
Keywords: EGFR protein, Human; Non‐small cell lung cancer; Pembrolizumab; United States Food and Drug Administration.
Published 2017. This article is a U.S. Government work and is in the public domain in the USA.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures
References
-
- D'Addario G, Pintilie M, Leighl NB et al. Platinum‐based versus non‐platinum based chemotherapy in advanced non‐small‐cell lung cancer: A meta‐analysis of the published literature. J Clin Oncol 2005;23:2926–2936. - PubMed
-
- Taxotere Prescribing Information . Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/020449s075lbl.pdf. Accessed July 19, 2017.
-
- Erlotinib Prescribing Information . Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021743s027lbl.pdf. Accessed July 19, 2017.
-
- National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology : Non‐small cell lung cancer. Available at https://www.nccn.org/professionals/physician_gls/pdf/nscl_blocks.pdf. Accessed December 12, 2016.
-
- National Cancer Institute : Lung Cancer. Available at https://www.cancer.gov/types/lung/hp/non-small-cell-lung-treatment-pdq. Accessed December 12, 2016.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

