The divisive normalization model of V1 neurons: a comprehensive comparison of physiological data and model predictions
- PMID: 28835531
- PMCID: PMC5814712
- DOI: 10.1152/jn.00821.2016
The divisive normalization model of V1 neurons: a comprehensive comparison of physiological data and model predictions
Abstract
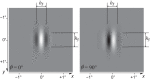
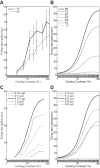
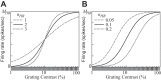
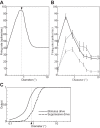
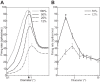
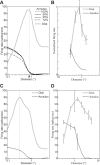
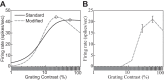
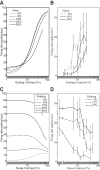
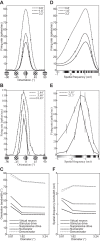
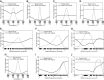
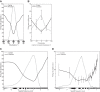
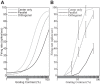
The physiological responses of simple and complex cells in the primary visual cortex (V1) have been studied extensively and modeled at different levels. At the functional level, the divisive normalization model (DNM; Heeger DJ. Vis Neurosci 9: 181-197, 1992) has accounted for a wide range of single-cell recordings in terms of a combination of linear filtering, nonlinear rectification, and divisive normalization. We propose standardizing the formulation of the DNM and implementing it in software that takes static grayscale images as inputs and produces firing rate responses as outputs. We also review a comprehensive suite of 30 empirical phenomena and report a series of simulation experiments that qualitatively replicate dozens of key experiments with a standard parameter set consistent with physiological measurements. This systematic approach identifies novel falsifiable predictions of the DNM. We show how the model simultaneously satisfies the conflicting desiderata of flexibility and falsifiability. Our key idea is that, while adjustable parameters are needed to accommodate the diversity across neurons, they must be fixed for a given individual neuron. This requirement introduces falsifiable constraints when this single neuron is probed with multiple stimuli. We also present mathematical analyses and simulation experiments that explicate some of these constraints.
Keywords: complex cells; computational modeling; divisive normalization; primary visual cortex (V1); simple cells.
Copyright © 2017 the American Physiological Society.
Figures











References
-
- Albrecht DG, Geisler WS, Crane AM. Nonlinear properties of visual cortex neurons: temporal dynamics, stimulus selectivity, neural performance. In: The Visual Neurosciences, edited by Chalupa L, Werner J. Boston, MA: MIT Press, 2003, p. 747–764.
-
- Albrecht DG, Geisler WS, Frazor RA, Crane AM. Visual cortex neurons of monkeys and cats: temporal dynamics of the contrast response function. J Neurophysiol 88: 888–913, 2002. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

