In vivo photothermal optical coherence tomography of endogenous and exogenous contrast agents in the eye
- PMID: 28835698
- PMCID: PMC5569082
- DOI: 10.1038/s41598-017-10050-5
In vivo photothermal optical coherence tomography of endogenous and exogenous contrast agents in the eye
Abstract
Optical coherence tomography (OCT) has become a standard-of-care in retinal imaging. OCT allows non-invasive imaging of the tissue structure but lacks specificity to contrast agents that could be used for in vivo molecular imaging. Photothermal OCT (PT-OCT) is a functional OCT-based technique that has been developed to detect absorbers in a sample. We demonstrate in vivo PT-OCT in the eye for the first time on both endogenous (melanin) and exogenous (gold nanorods) absorbers. Pigmented mice and albino mice (n = 6 eyes) were used to isolate the photothermal signal from the melanin in the retina. Pigmented mice with laser-induced choroidal neovascularization lesions (n = 7 eyes) were also imaged after a systemic injection of gold nanorods to observe their passive accumulation in the retina. This experiment demonstrates the feasibility of PT-OCT to image the distribution of both endogenous and exogenous absorbers in the mouse retina.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
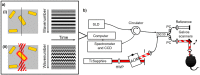
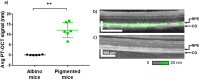
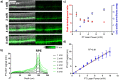
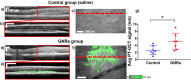
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

