Glycoprotein 2 is a specific cell surface marker of human pancreatic progenitors
- PMID: 28835709
- PMCID: PMC5569081
- DOI: 10.1038/s41467-017-00561-0
Glycoprotein 2 is a specific cell surface marker of human pancreatic progenitors
Abstract
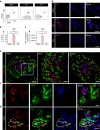
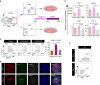
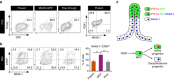
PDX1+/NKX6-1+ pancreatic progenitors (PPs) give rise to endocrine cells both in vitro and in vivo. This cell population can be successfully differentiated from human pluripotent stem cells (hPSCs) and hold the potential to generate an unlimited supply of β cells for diabetes treatment. However, the efficiency of PP generation in vitro is highly variable, negatively impacting reproducibility and validation of in vitro and in vivo studies, and consequently, translation to the clinic. Here, we report the use of a proteomics approach to phenotypically characterize hPSC-derived PPs and distinguish these cells from non-PP populations during differentiation. Our analysis identifies the pancreatic secretory granule membrane major glycoprotein 2 (GP2) as a PP-specific cell surface marker. Remarkably, GP2 is co-expressed with NKX6-1 and PTF1A in human developing pancreata, indicating that it marks the multipotent pancreatic progenitors in vivo. Finally, we show that isolated hPSC-derived GP2+ cells generate β-like cells (C-PEPTIDE+/NKX6-1+) more efficiently compared to GP2- and unsorted populations, underlining the potential therapeutic applications of GP2.Pancreatic progenitors (PPs) can be derived from human pluripotent stem cells in vitro but efficiency of differentiation varies, making it hard to sort for insulin-producing cells. Here, the authors use a proteomic approach to identify the secretory granule membrane glycoprotein 2 as a marker for PDX1+/NKX6-1+ PPs.
Conflict of interest statement
The method utilized to create the PP cells is patented and under license to Sernova Inc. and M.C.N. and F.S. are inventors on this patent. OHSU has commercially licensed some of the technology described herein (HPx1, HPx2); authors C.D., P.R.S., and M.G. are inventors of these antibodies. This potential conflict of interest has been reviewed and managed by OHSU. The remaining authors declare no competing financial interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

