Checkpoint immunotherapy in head and neck cancers
- PMID: 28836124
- PMCID: PMC7694874
- DOI: 10.1007/s10555-017-9694-9
Checkpoint immunotherapy in head and neck cancers
Abstract
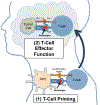
Checkpoint inhibitors have recently gained FDA approval for the treatment of cisplatin-resistant recurrent and metastatic head and neck squamous cell carcinoma (HNSCC) by outperforming standard of care chemotherapy and inducing durable responses in a subset of patients. These monoclonal antibodies unleash the patient's own immune system to target cancer cells. HNSCC is a good target for these agents as there is ample evidence of active immunosurveillance in the head and neck and a number of immune evasion mechanisms by which HNSCCs form progressive disease including via the PD-1/PD-L1 axis. As HNSCCs typically possess a moderately high mutation burden, they should express numerous mutation-derived antigen targets for immune detection. However, with response rates less than 20% in clinical trials, there is a need for biomarkers to screen patients as well as clinical trials evaluating novel combinations to improve outcomes. The aim of this review is to provide historical and mechanistic context for the use of checkpoint inhibitors in head and neck cancer and provide a perspective on the role of novel checkpoints, biomarkers, and combination therapies that are evolving in the near term for patients with HNSCC.
Keywords: Checkpoint blockade; Head and neck squamous cell carcinoma; Immunotherapy.
Figures
References
-
- Chow LQ, Haddad R, Gupta S, Mahipal A, Mehra R, Tahara M, et al. (2016). Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: Results from the phase Ib KEYNOTE-012 expansion cohort. Journal of Clinical Oncology, 34(32), 3838–3845. - PMC - PubMed
-
- Whiteside TL, Letessier E, Hirabayashi H, Vitolo D, Bryant J, Barnes L, et al. (1993). Evidence for local and systemic activation of immune cells by peritumoral injections of interleukin 2 in patients with advanced squamous cell carcinoma of the head and neck. Cancer research, 53(23), 5654–5662. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials