Late (≥ 7 days) inhalation corticosteroids to reduce bronchopulmonary dysplasia in preterm infants
- PMID: 28836266
- PMCID: PMC6483527
- DOI: 10.1002/14651858.CD002311.pub4
Late (≥ 7 days) inhalation corticosteroids to reduce bronchopulmonary dysplasia in preterm infants
Update in
-
Late (≥ 7 days) inhaled corticosteroids to reduce bronchopulmonary dysplasia in preterm infants.Cochrane Database Syst Rev. 2022 Dec 15;12(12):CD002311. doi: 10.1002/14651858.CD002311.pub5. Cochrane Database Syst Rev. 2022. PMID: 36521169 Free PMC article.
Abstract
Background: Bronchopulmonary dysplasia (BPD), defined as oxygen dependence at 36 weeks postmenstrual age (PMA), remains an important complication of prematurity. Pulmonary inflammation plays a central role in the pathogenesis of BPD. Attenuating pulmonary inflammation with postnatal systemic corticosteroids reduces the incidence of BPD in preterm infants but may be associated with an increased risk of adverse neurodevelopmental outcomes. Local administration of corticosteroids via inhalation might be an effective and safe alternative.
Objectives: To determine if administration of inhalation corticosteroids after the first week of life until 36 weeks PMA to preterm infants at high risk of developing BPD is effective and safe in reducing the incidence of death and BPD as separate or combined outcomes.
Search methods: We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL 2017, Issue 4), MEDLINE via PubMed (1966 to 19 May 2017), Embase (1980 to 19 May 2017), and CINAHL (1982 to 19 May 2017). We also searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles for randomised controlled trials and quasi-randomised trials.
Selection criteria: We included randomised controlled trials comparing inhalation corticosteroids, started ≥ 7 days postnatal age (PNA) but before 36 weeks PMA, to placebo in ventilated and non-ventilated infants at risk of BPD. We excluded trials investigating systemic corticosteroids versus inhalation corticosteroids.
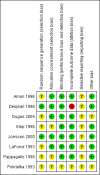
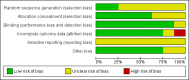
Data collection and analysis: We collected data on participant characteristics, trial methodology, and inhalation regimens. The primary outcome was death or BPD at 36 weeks PMA. Secondary outcomes were the combined outcome death or BPD at 28 days PNA, the seperate outcomes of death and BPD at both 28 days PNA, and at 36 weeks PMA, and short-term respiratory outcomes, such as failure to extubate; total days of mechanical ventilation and oxygen use; and the need for systemic corticosteroids. We contacted the original trialists to verify the validity of extracted data and to provide missing data. We analysed all data using Review Manager 5. When possible, we performed meta-analysis using typical risk ratio (RR) for dichotomous outcomes and weighted mean difference (WMD) for continuous outcomes along with their 95% confidence intervals (CI). We analysed ventilated and non-ventilated participants separately.We used the GRADE approach to assess the quality of the evidence.
Main results: We included eight trials randomising 232 preterm infants in this review. Inhalation corticosteroids did not reduce the separate or combined outcomes of death or BPD. The meta-analyses of the studies showed a reduced risk in favor of inhalation steroids regarding failure to extubate at seven days (typical RR (TRR) 0.80, 95% CI 0.66 to 0.98; 5 studies, 79 infants) and at the latest reported time point after treatment onset (TRR 0.60, 95% CI 0.45 to 0.80; 6 studies, 90 infants). However, both analyses showed increased statistical heterogeneity (I2 statistic 73% and 86%, respectively). Furthermore, inhalation steroids did not impact total duration of mechanical ventilation or oxygen dependency. There was a trend toward a reduction in the use of systemic corticosteroids in infants receiving inhalation corticosteroids (TRR 0.51, 95% CI 0.26 to 1.00; 4 studies, 74 infants; very low-quality evidence). There was a paucity of data on short- and long-term adverse effects. Our results should be interpreted with caution because the total number of randomised participants is relatively small, and most trials differed considerably in participant characteristics, inhalation therapy, and outcome definitions.
Authors' conclusions: Based on the results of the currently available evidence, inhalation corticosteroids initiated at ≥ 7 days of life for preterm infants at high risk of developing BPD cannot be recommended at this point in time. More and larger randomised, placebo-controlled trials are needed to establish the efficacy and safety of inhalation corticosteroids.
Conflict of interest statement
Wes Onland: No financial disclosure to be declared. No potential conflicts of interest known. Martin Offringa: No financial disclosure to be declared. No potential conflicts of interest known. Anton van Kaam: No financial disclosure to be declared. No potential conflicts of interest known.
Figures
Update of
-
Late (≥ 7 days) inhalation corticosteroids to reduce bronchopulmonary dysplasia in preterm infants.Cochrane Database Syst Rev. 2012 Apr 18;(4):CD002311. doi: 10.1002/14651858.CD002311.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2017 Aug 24;8:CD002311. doi: 10.1002/14651858.CD002311.pub4. PMID: 22513906 Updated.
References
References to studies included in this review
Arnon 1996 {published and unpublished data}
Denjean 1998 {published data only (unpublished sought but not used)}
-
- Denjean A, Paris‐Llado J, Zupan V, Debillon T, Kieffer F, Magny JF, et al. Inhaled salbutamol and beclomethasone for preventing broncho‐pulmonary dysplasia: a randomised double‐blind study. European Journal of Pediatrics 1998;157(11):926‐31. [PUBMED: 9835439] - PubMed
Dugas 2005 {published and unpublished data}
-
- Dugas MA, Nguyen D, Frenette L, Lachance C, Belanger S, Caouette G, et al. Clinical outcome of inhaled fluticasone in moderate bronchopulmonary dysplasia. American Journal of Respiratory Critical Care Medicine 2003;167:A385.
-
- Piedboeuf B, Dugas MA, Nguyen D, Frenette L, Proulx E, Lachance C. Clinical outcome of inhaled fluticasone in moderate bronchopulmonary dysplasia. Pediatric Research 2003;53:411A. - PubMed
Giep 1996 {published data only (unpublished sought but not used)}
-
- Schwartz ID, Giep T. Adrenal function in preterm infants treated with beclomethasone. Journal of Pediatrics 2000;136(6):851‐2. [PUBMED: 10839892] - PubMed
Jonsson 2000 {published and unpublished data}
-
- Jonsson B, Eriksson M, Soder O, Broberger U, Lagercrantz H. Budesonide delivered by dosimetric jet nebulization to preterm very low birthweight infants at high risk for development of chronic lung disease. Acta Paediatrica 2000;89(12):1449‐55. [PUBMED: 11195235] - PubMed
LaForce 1993 {published and unpublished data}
-
- LaForce WR, Brudno DS. Controlled trial of beclomethasone dipropionate by nebulization in oxygen‐ and ventilator‐dependent infants. Journal of Pediatrics 1993;122(2):285‐8. [PUBMED: 8429447] - PubMed
Pappagallo 1998 {published data only (unpublished sought but not used)}
-
- Pappagallo M, Abbasi S, Bhutani VK. Respiratory and systemic effects of inhaled dexamethasone on ventilator dependent preterm infants at risk for bronchopulmonary dysplasia. Indian Journal of Pediatrics 1998;65(2):273‐82. [PUBMED: 10771973] - PubMed
-
- Pappagallo M, Bhutani V, Abbasi S. Nebulised steroid trial in ventilator‐dependent preterm infants. Pediatric Research 1991;29:327A.
Pokriefka 1993 {published data only (unpublished sought but not used)}
-
- Pokriefka E, Mehdizadeh B, Rabbani A. Inhaled flunisolide in bronchopulmonary dysplasia. Pediatric Research 1993;33:341A.
References to studies excluded from this review
Abbasi 1993 {published data only}
-
- Abbasi S, Silva W, Gerdes JS, Bhutani VK. Role of dexamethasone therapy for severe trachebronchial mucosal lesions in mechanically ventilated preterm infants. Pediatric Research 1993; Vol. 33:198A.
Beresford 2002 {published data only}
Cloutier 1993 {published data only}
-
- Cloutier M. Nebulized steroid therapy in bronchopulmonary dysplasia. Pediatric Pulmonology 1993;15(2):111‐6. [PUBMED: 8474782] - PubMed
Cole 1999a {published data only}
-
- Cole CH, Abbasi S, Dorkin H, Shah B, Demissie S, Mackinnon B, et al. Early inhaled glucocorticoid therapy for prevention of BPD: assessment of growth, history, and pulmonary function at 6 months corrected age. Pediatric Research 1999;45:190A.
-
- Cole CH, Shah B, Abbasi S, Demissie S, Mackinnon B, Colton T, et al. Adrenal function in premature infants during inhaled beclomethasone therapy. Journal of Pediatrics 1999;135(1):65‐70. [PUBMED: 10393606] - PubMed
-
- Gupta GK, Cole CH, Abbasi S, Demissie S, Njinimbam C, Nielsen HC, et al. Effects of early inhaled beclomethasone therapy on tracheal aspirate inflammatory mediators IL‐8 and IL‐1ra in ventilated preterm infants at risk for bronchopulmonary dysplasia. Pediatric Pulmonology 2000;30(4):275‐81. [PUBMED: 11015126] - PubMed
-
- Gupta GK, Cole CH, Abbasi S, Nielsen HC, Demissie S, Colton T, et al. Effect of beclomethasone therapy on tracheal aspirate interleukine‐8 and interleukine‐1 receptor antagonist in preterm ventilated infants at risk for bronchopulmonary dysplasia (BPD). Pediatric Research 1998; Vol. 45:303A. - PubMed
Dimitriou 1997 {published data only}
-
- Dimitriou G, Greenough A, Giffin F, Kavadia V. Inhaled versus systemic steroids in chronic oxygen dependency of preterm infants. European Journal of Pediatrics 1997;156(1):51‐5. - PubMed
Dunn 1989 {published data only}
-
- Dunn MS, Magnani L, Belaiche M. Inhaled corticosteroids in severe bronchopulmonary dysplasia. Pediatric Research 1989;25:213A.
Dunn 1992 {published data only}
-
- Dunn M, Pandit P, Magnani L, Anaka R, Kirpalani H. Inhaled corticosteroids for neonatal chronic lung disease: a randomised, double‐blind cross‐over study. Pediatric Research 1992;31:201A.
Eisenberg 1990 {published data only}
-
- Eisenberg J. Use of nebulized flunisolide in infants and children with asthma and bronchopulmonary dysplasia. American Review of Respiratory Disease 1990;141:A899.
Giffin 1994 {published data only}
-
- Giffin F, Greenough A. A pilot study assessing inhaled budesonide in chronically oxygen‐dependent infants. Acta Paediatrica 1994;83(6):669‐71. [PUBMED: 7919769] - PubMed
Inwald 1999 {published data only}
-
- Inwald DP, Trivedi K, Murch SH, Costeloe K. The effect of early inhaled budesonide on pulmonary inflammation in infants with respiratory distress syndrome. European Journal of Pediatrics 1999;158(10):815‐6. [PUBMED: 10486083] - PubMed
Konig 1992 {published data only}
Kovacs 1998 {published data only}
-
- Kovacs L, Davis GM, Faucher D, Papageorgiou A. Efficacy of sequential early systemic and inhaled corticosteroid therapy in the prevention of chronic lung disease of prematurity. Acta Paediatrica 1998;87(7):792‐8. [PUBMED: 9722255] - PubMed
-
- Kovacs L, Davis M, Faucher DJ, Papageorgiou AN. Efficacy of early systemic and inhaled corticosteroid therapy in the prevention of chronic lung disease of prematurity. Pediatric Research 1996;39:337A. - PubMed
Kugelman 2017 {published data only}
-
- Kugelman A, Peniakov M, Zangen S, Shiff Y, Riskin A, Iofe A, et al. Inhaled hydrofluoalkane‐beclomethasone dipropionate in bronchopulmonary dysplasia. A double‐blind, randomized, controlled pilot study. Journal of Perinatology 2017;37(2):197‐202. [DOI: 10.1038/jp.2016.177; PUBMED: 27735931] - DOI - PubMed
Liu 1996 {published data only}
-
- Liu EA, Heldt GP. A trial of the safety of inhaled beclomethasone in ventilator‐treated neonates. Journal of Pediatrics 1996;129(1):154‐6. [PUBMED: 8757577] - PubMed
-
- Liu EA, Heldt GP. Effects of inhaled beclomethasone in intubated neonates: phase 1 trial. Pediatric Research 1993;33:334A.
Ng 1998 {published data only}
-
- Ng PC, Fok TF, Wong GW, Lam CW, Lee CH, Wong MY, et al. Pituitary‐adrenal suppression in preterm, very low birth weight infants after inhaled fluticasone propionate treatment. Journal of Clinical Endocrinology and Metabolism 1998;83(7):2390‐3. [DOI: 10.1210/jcem.83.7.4947; PUBMED: 9661616] - DOI - PubMed
Pappagallo 1990 {published data only}
-
- Pappagallo M, Blondheim O, Bhutani VK, Abbasi S. Effect of inhaled dexamethasone in ventilator dependent preterm infants. Pediatric Research 1990; Vol. 27:219A. - PubMed
Parikh 2002 {published data only}
-
- Parikh NA, Locke RG, Chidekel A, Leef KH, Emberger J, Paul DA, et al. Effect of inhaled corticosteroids on inflammatory cytokines in preterm infants with evolving chronic lung disease. Pediatric Research 2002;51:356A. - PubMed
-
- Parikh NA, Locke RG, Chidekel A, Leef KH, Emberger J, Paul DA, et al. Effect of inhaled corticosteroids on markers of pulmonary inflammation and lung maturation in preterm infants with evolving chronic lung disease. Journal of the American Osteopathic Association 2004;104(3):114‐20. [PUBMED: 15083986] - PubMed
Pelkonen 2001 {published data only}
Rajamani 1998 {published data only}
-
- Rajamani S, Dothey C, Super D, Martin J. Early inhaled beclomethasone does not alter the course of lung disease in very low birth weight infants (VLBW) at risk of bronchopulmonary dysplasia (BPD). Pediatric Research 1998;45:219A.
Rozycki 2003 {published data only}
-
- Rozycki HJ, Byron PR, Elliott GR, Carroll T, Gutcher GR. Randomized controlled trial of three different doses of aerosol beclomethasone versus systemic dexamethasone to promote extubation in ventilated premature infants. Pediatric Pulmonology 2003;35(5):375‐83. - PubMed
Shah 2007 {published data only}
-
- Jangaard K, Vincer MJ, Dunn M, Asztalos E, Shah VS. Neurodevelopmental outcomes in ventilated preterm infants < 1250 grams enrolled in a dose‐ranging study assessing the effect of inhaled corticosteroids. Pediatric Academic Societies (PAS) 2009 Annual Meeting; 2009 May 02 ‐ 05; Baltimore (MD). Baltimore (MD), 2009. [4347.301]
-
- Shah VS, Jangaard K, Reilly M, Dunn M. Inhaled corticosteroids in ventilated preterm infants: an escalating dose‐ranging study. Pediatric Academic Societies (PAS) 2007 Annual Meeting; 2007 May 03 ‐ 08; Toronto (ON). Toronto (ON), 2007:E‐PAS2007:7935.21. [E‐PAS2007:7935.21]
Suchomski 1996 {published data only}
-
- Suchomski S, Cummings J. Randomised trial of inhaled vs intravenous steroid in ventilator dependent preterm infants. Pediatric Research 1996;39:247A. - PubMed
Thorson 1992 {published data only}
-
- Gal P, Diaz PR, Ransom JL, Carlos RQ, Thorson DW. Beclomethasone for treating premature infants with bronchopulmonary dysplasia. Journal of Pediatrics 1993;123(3):490‐1. [PUBMED: 8355132] - PubMed
-
- Thorson DW, Gal P, Diaz PR, Ransom JL, Weaver RL, Carlos RQ. Beclomethasone MDI for treating bronchopulmonary dysplasia in premature infants: a preliminary report. Pharmacotherapy 1992;12:261.
Yeh 2008 {published data only}
Additional references
Bolton 2015
Cheong 2013
-
- Cheong JL, Anderson P, Roberts G, Duff J, Doyle LW, Victorian Infant Collaborative Study Group. Postnatal corticosteroids and neurodevelopmental outcomes in extremely low birthweight or extremely preterm infants: 15‐year experience in Victoria, Australia. Archives of Disease in Childhood. Fetal and Neonatal Edition 2013;98(1):F32‐6. [DOI: 10.1136/fetalneonatal-2011-301355; PUBMED: 22684163] - DOI - PubMed
Davis 2001
Doyle 2006
Doyle 2014
Doyle 2014a
Fok 1996
-
- Fok TF, Monkman S, Dolovich M, Gray S, Coates G, Paes B, et al. Efficiency of aerosol medication delivery from a metered dose inhaler versus jet nebulizer in infants with bronchopulmonary dysplasia. Pediatric Pulmonology 1996;21(5):301‐9. [DOI: 10.1002/(SICI)1099-0496(199605)21:5%3C301::AID-PPUL5%3E3.0.CO;2-P; PUBMED: 8726155] - DOI - PubMed
GRADEpro GDT [Computer program]
-
- GRADE Working Group, McMaster University. GRADEpro GDT. Version (accessed 19 May 2017). Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Higgins 2011
-
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Ibrahim 2011
Job 2015
Jobe 2001
-
- Jobe AH, Bancalari E. Bronchopulmonary dysplasia. American Journal of Respiratory and Critical Care Medicine 2001;163(7):1723‐9. [DOI: ; PUBMED: 10.1164/ajrccm.163.7.2011060] - PubMed
Maas 2010
McEvoy 2014
-
- McEvoy CT, Jain L, Schmidt B, Abman S, Bancalari E, Aschner JL. Bronchopulmonary dysplasia: NHLBI Workshop on the Primary Prevention of Chronic Lung Diseases. Annals of the American Thoracic Society 2014;11(Suppl 3):S146‐53. [DOI: 10.1513/AnnalsATS.201312-424LD; PUBMED: 24754823] - DOI - PMC - PubMed
Ogawa 2015
Onland 2017
Pierce 1995
-
- Pierce MR, Bancalari E. The role of inflammation in the pathogenesis of bronchopulmonary dysplasia. Pediatric Pulmonology 1995;19(6):371‐8. [PUBMED: 7567218] - PubMed
RevMan 5 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A (editors), GRADE Working Group. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. gdt.gradepro.org/app/handbook/handbook.html (accessed prior to 1 August 2017).
Shah 2012
Shah 2012a
-
- Shah SS, Ohlsson A, Halliday HL, Shah VS. Inhaled versus systemic corticosteroids for the treatment of chronic lung disease in ventilated very low birth weight preterm infants. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD002057.pub3; PUBMED: 22592682] - DOI - PubMed
Shah 2017
Short 2007
-
- Short EJ, Kirchner HL, Asaad GR, Fulton SE, Lewis BA, Klein N, et al. Developmental sequelae in preterm infants having a diagnosis of bronchopulmonary dysplasia: analysis using a severity‐based classification system. Archives of Pediatrics and Adolescent Medicine 2007;161(11):1082‐7. [DOI: 10.1001/archpedi.161.11.1082; PUBMED: 17984411] - DOI - PMC - PubMed
Slaughter 2014
Stoll 2010
-
- Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010;126(3):443‐56. [DOI: 10.1542/peds.2009-2959; PUBMED: 20732945] - DOI - PMC - PubMed
Walsh 2005
-
- Walsh MC, Morris BH, Wrage LA, Vohr BR, Poole WK, Tyson JE, et al. National Institute of Child Health and Human Development Neonatal Research Network. Extremely low birthweight neonates with protracted ventilation: mortality and 18‐month neurodevelopmental outcomes. Journal of Pediatrics 2005;146(6):798‐804. [DOI: 10.1016/j.jpeds.2005.01.047; PUBMED: 15973322] - DOI - PubMed
Walsh 2006
Ward 2003
References to other published versions of this review
Lister 2000
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

