Transient exposure to oxygen or nitrate reveals ecophysiology of fermentative and sulfate-reducing benthic microbial populations
- PMID: 28836729
- PMCID: PMC5763382
- DOI: 10.1111/1462-2920.13895
Transient exposure to oxygen or nitrate reveals ecophysiology of fermentative and sulfate-reducing benthic microbial populations
Abstract
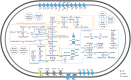
For the anaerobic remineralization of organic matter in marine sediments, sulfate reduction coupled to fermentation plays a key role. Here, we enriched sulfate-reducing/fermentative communities from intertidal sediments under defined conditions in continuous culture. We transiently exposed the cultures to oxygen or nitrate twice daily and investigated the community response. Chemical measurements, provisional genomes and transcriptomic profiles revealed trophic networks of microbial populations. Sulfate reducers coexisted with facultative nitrate reducers or aerobes enabling the community to adjust to nitrate or oxygen pulses. Exposure to oxygen and nitrate impacted the community structure, but did not suppress fermentation or sulfate reduction as community functions, highlighting their stability under dynamic conditions. The most abundant sulfate reducer in all cultures, related to Desulfotignum balticum, appeared to have coupled both acetate- and hydrogen oxidation to sulfate reduction. We describe a novel representative of the widespread uncultured candidate phylum Fermentibacteria (formerly candidate division Hyd24-12). For this strictly anaerobic, obligate fermentative bacterium, we propose the name 'U Sabulitectum silens' and identify it as a partner of sulfate reducers in marine sediments. Overall, we provide insights into the function of fermentative, as well as sulfate-reducing microbial communities and their adaptation to a dynamic environment.
© 2017 The Authors. Environmental Microbiology published by Society for Applied Microbiology and John Wiley & Sons Ltd.
Figures





References
-
- Aller, R.C. (1994) Bioturbation and remineralization of sedimentary organic matter: effects of redox oscillation. Chem Geol 114: 331–345.
-
- Barton, L.L. , and Fauque, G.D. (2009) Chapter 2: biochemistry, physiology and biotechnology of sulfate‐reducing bacteria. Adv Appl Microbiol 68: 41–98. - PubMed
-
- van Beusekom, J.E.E. (2005) A historic perspective on Wadden Sea eutrophication. Helgol Mar Res 59: 45–54.
-
- Billerbeck, M. , Werner, U. , Polerecky, L. , Walpersdorf, E. , deBeer, D. , and Huettel, M. (2006) Surficial and deep pore water circulation governs spatial and temporal scales of nutrient recycling in intertidal sand flat sediment. Mar Ecol Prog Ser 326: 61–76.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

