The feasibility and benefit of a brief psychosocial intervention in addition to early palliative care in patients with advanced cancer to reduce depressive symptoms: a pilot randomized controlled clinical trial
- PMID: 28836960
- PMCID: PMC5569457
- DOI: 10.1186/s12885-017-3560-6
The feasibility and benefit of a brief psychosocial intervention in addition to early palliative care in patients with advanced cancer to reduce depressive symptoms: a pilot randomized controlled clinical trial
Abstract
Background: The aim of this study was to assess the feasibility and potential benefit of a brief psychosocial intervention based on cognitive-behavioral therapy performed in addition to early palliative care (PC) in the reduction of depressive symptoms among patients with advanced cancer.
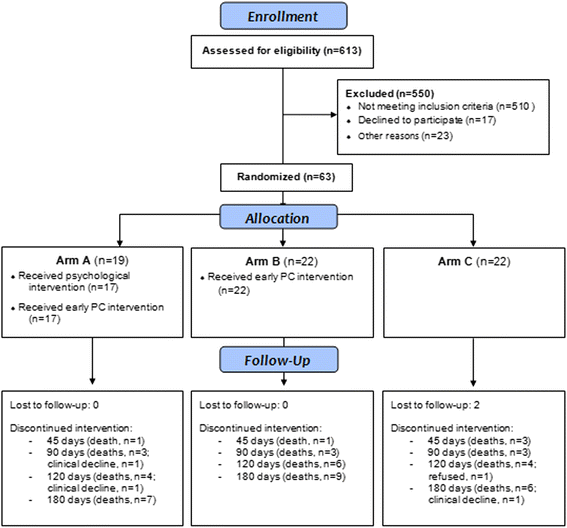
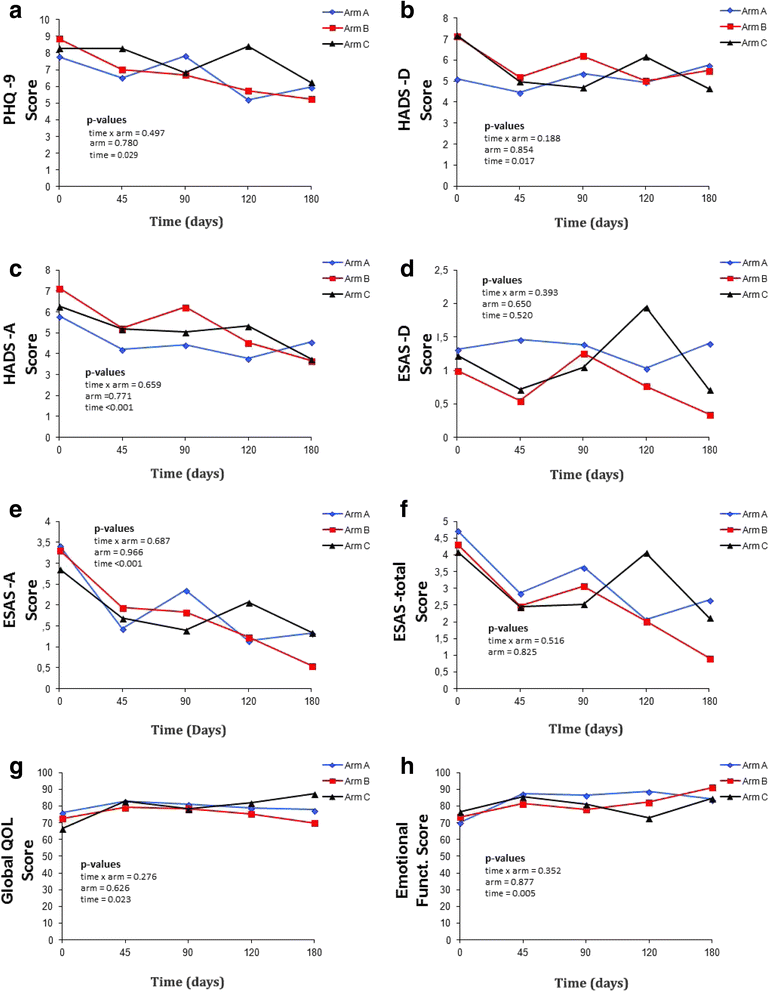
Methods: An open-label randomized phase II clinical trial with two intervention arms and one control group. Patients with advanced cancer starting palliative chemotherapy and who met the selection criteria were included. The participants were randomly allocated to three arms: arm A, five weekly sessions of psychosocial intervention combined with early PC; arm B, early PC only; and arm C, standard cancer treatment. Feasibility was investigated by calculating rates (%) of inclusion, attrition, and contamination (% of patients from Arm C that received PC). Scores of depression (primary aim), anxiety, and quality of life were measured at baseline and 45, 90, 120, and 180 days after randomization.
Results: From the total of 613 screened patients (10.3% inclusion rate), 19, 22, and 22 patients were allocated to arms A, B, and C, respectively. Contamination and attrition rates (180 days) were 31.8% and 38.0%, respectively. No interaction between the arms and treatments were found. Regarding effect sizes, there was a moderate benefit in arm A over arms B and C in emotional functioning (-0.66 and -0.61, respectively) but a negative effect of arm A over arm C in depression (-0.74).
Conclusions: Future studies to be conducted with this population group need to revise the eligibility criteria and make them less restrictive. In addition, the need for arm C is questioned due to high contamination rate. The designed psychosocial intervention was not able to reduce depressive symptoms when combined with early PC. Further studies are warrant to evaluate the intervention on-demand and in subgroups of high risk of anxiety/depression.
Trial registration: Clinical Trials identifier NCT02133274 . Registered May 6, 2014.
Keywords: Anxiety; Cognitive therapy; Depression; Neoplasms; Palliative care.
Conflict of interest statement
Ethics approval and consent to participate
The research protocol for this study was developed according to the standards of the National Health Council Resolution number 466/12 and the guidelines of the Declaration of Helsinki. The study protocol was approved by the Research Ethics Committee of the Barretos Cancer Hospital (CEP/HCB n° 699/014) and all participants signed a free and informed consent form.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- LeBlanc TW, Nickolich MS, El-Jawahri A, Temel JS. Discussing the Evidence for Upstream Palliative Care in Improving Outcomes in Advanced Cancer. Am. Soc. Clin. Oncol. Educ. B. [Internet]. 2016 [cited 2016 Nov 29];36:e534–8. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

