Whole Genome Sequencing Revealed Mutations in Two Independent Genes as the Underlying Cause of Retinal Degeneration in an Ashkenazi Jewish Pedigree
- PMID: 28837078
- PMCID: PMC5615344
- DOI: 10.3390/genes8090210
Whole Genome Sequencing Revealed Mutations in Two Independent Genes as the Underlying Cause of Retinal Degeneration in an Ashkenazi Jewish Pedigree
Erratum in
-
Correction: Gustafson et al., Whole Genome Sequencing Revealed Mutations in Two Independent Genes as the Underlying Cause of Retinal Degeneration in an Ashkenazi Jewish Pedigree. Genes 2017, 8, 210.Genes (Basel). 2017 Oct 23;8(10):286. doi: 10.3390/genes8100286. Genes (Basel). 2017. PMID: 29065517 Free PMC article.
Abstract
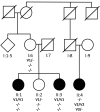
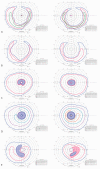
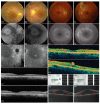
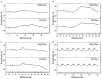
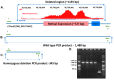
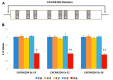
Retinitis pigmentosa (RP) causes progressive photoreceptor loss resulting from mutations in over 80 genes. This study identified the genetic cause of RP in three members of a non-consanguineous pedigree. Detailed ophthalmic evaluation was performed in the three affected family members. Whole exome sequencing (WES) and whole genome sequencing (WGS) were performed in the three affected and the two unaffected family members and variants were filtered to detect rare, potentially deleterious variants segregating with disease. WES and WGS did not identify potentially pathogenic variants shared by all three affected members. However, WES identified a previously reported homozygous nonsense mutation in KIZ (c.226C>T, p.Arg76*) in two affected sisters, but not in their affected second cousin. WGS revealed a novel 1.135 kb homozygous deletion in a retina transcript of C21orf2 and a novel 30.651 kb heterozygous deletion in CACNA2D4 in the affected second cousin. The sisters with the KIZ mutation carried no copies of the C21orf2 or CACNA2D4 deletions, while the second cousin with the C21orf2 and CACNA2D4 deletions carried no copies of the KIZ mutation. This study identified two independent, homozygous mutations in genes previously reported in autosomal recessive RP in a non-consanguineous family, and demonstrated the value of WGS when WES fails to identify likely disease-causing mutations.
Keywords: genetics; retina; retinitis pigmentosa.
Conflict of interest statement
All authors declare no conflict of interest except Amalio Telenti is an employee of Human Longevity, Inc. and Radha Ayyagari’s spouse is an employee of Pfizer. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures
Similar articles
-
Genetic and Clinical Analyses of the KIZ-c.226C>T Variant Resulting in a Dual Mutational Mechanism.Genes (Basel). 2024 Jun 18;15(6):804. doi: 10.3390/genes15060804. Genes (Basel). 2024. PMID: 38927740 Free PMC article.
-
Whole-exome sequencing identifies KIZ as a ciliary gene associated with autosomal-recessive rod-cone dystrophy.Am J Hum Genet. 2014 Apr 3;94(4):625-33. doi: 10.1016/j.ajhg.2014.03.005. Epub 2014 Mar 27. Am J Hum Genet. 2014. PMID: 24680887 Free PMC article.
-
Non-homologous recombination between Alu and LINE-1 repeats results in a 91 kb deletion in MERTK causing severe retinitis pigmentosa.Mol Vis. 2018 Oct 18;24:667-678. eCollection 2018. Mol Vis. 2018. PMID: 30416333 Free PMC article.
-
Exome sequencing extends the phenotypic spectrum for ABHD12 mutations: from syndromic to nonsyndromic retinal degeneration.Ophthalmology. 2014 Aug;121(8):1620-7. doi: 10.1016/j.ophtha.2014.02.008. Epub 2014 Mar 31. Ophthalmology. 2014. PMID: 24697911
-
OR2W3 sequence variants are unlikely to cause inherited retinal diseases.Ophthalmic Genet. 2016 Dec;37(4):366-368. doi: 10.3109/13816810.2015.1081252. Epub 2016 Feb 18. Ophthalmic Genet. 2016. PMID: 26891008 Review.
Cited by
-
Selection Signatures in Italian Livestock Guardian and Herding Shepherd Dogs.Vet Sci. 2022 Dec 21;10(1):3. doi: 10.3390/vetsci10010003. Vet Sci. 2022. PMID: 36669004 Free PMC article.
-
Expanding the genotypic and phenotypic spectra with a novel variant in the ciliopathy gene, CFAP410, associated with selective cone degeneration.Ophthalmic Genet. 2024 Dec;45(6):633-639. doi: 10.1080/13816810.2024.2369271. Epub 2024 Sep 4. Ophthalmic Genet. 2024. PMID: 39232248 Free PMC article.
-
Sensing through Non-Sensing Ocular Ion Channels.Int J Mol Sci. 2020 Sep 21;21(18):6925. doi: 10.3390/ijms21186925. Int J Mol Sci. 2020. PMID: 32967234 Free PMC article. Review.
-
C21orf2 variants causing inherited retinal disease: A review of what we know and a report of two new suspected cases.Clin Case Rep. 2023 Mar 19;11(3):e7110. doi: 10.1002/ccr3.7110. eCollection 2023 Mar. Clin Case Rep. 2023. PMID: 36950666 Free PMC article.
-
Genetic and Clinical Analyses of the KIZ-c.226C>T Variant Resulting in a Dual Mutational Mechanism.Genes (Basel). 2024 Jun 18;15(6):804. doi: 10.3390/genes15060804. Genes (Basel). 2024. PMID: 38927740 Free PMC article.
References
-
- Retnet. [(accessed on 12 May 2017)]; Available online: www.sph.uth.tmc.edu/RetNet/
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

