RNA Interference and BMP-2 Stimulation Allows Equine Chondrocytes Redifferentiation in 3D-Hypoxia Cell Culture Model: Application for Matrix-Induced Autologous Chondrocyte Implantation
- PMID: 28837082
- PMCID: PMC5618491
- DOI: 10.3390/ijms18091842
RNA Interference and BMP-2 Stimulation Allows Equine Chondrocytes Redifferentiation in 3D-Hypoxia Cell Culture Model: Application for Matrix-Induced Autologous Chondrocyte Implantation
Abstract
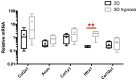
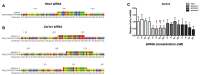
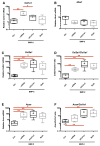
As in humans, osteoarthritis (OA) causes considerable economic loss to the equine industry. New hopes for cartilage repair have emerged with the matrix-associated autologous chondrocyte implantation (MACI). Nevertheless, its limitation is due to the dedifferentiation occurring during the chondrocyte amplification phase, leading to the loss of its capacity to produce a hyaline extracellular matrix (ECM). To enhance the MACI therapy efficiency, we have developed a strategy for chondrocyte redifferentiation, and demonstrated its feasibility in the equine model. Thus, to mimic the cartilage microenvironment, the equine dedifferentiated chondrocytes were cultured in type I/III collagen sponges for 7 days under hypoxia in the presence of BMP-2. In addition, chondrocytes were transfected by siRNA targeting Col1a1 and Htra1 mRNAs, which are overexpressed during dedifferentiation and OA. To investigate the quality of the neo-synthesized ECM, specific and atypical cartilage markers were evaluated by RT-qPCR and Western blot. Our results show that the combination of 3D hypoxia cell culture, BMP-2 (Bone morphogenetic protein-2), and RNA interference, increases the chondrocytes functional indexes (Col2a1/Col1a1, Acan/Col1a1), leading to an effective chondrocyte redifferentiation. These data represent a proof of concept for this process of application, in vitro, in the equine model, and will lead to the improvement of the MACI efficiency for cartilage tissue engineering therapy in preclinical/clinical trials, both in equine and human medicine.
Keywords: BMP-2; Col1a1; HtrA1; chondrocyte; dedifferentiation; equine model; hypoxia; matrix-associated autologous chondrocyte implantation (MACI); siRNA; tissue engineering; type II collagen.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Oke S.L., McIlwraith C.W. Review of the economic impact of osteoarthritis and oral joint-health supplements in horses; Proceedings of the 56th Annual Convention of the American Association of Equine Practitioners; Baltimore, MD, USA. 4–8 December 2010; pp. 12–16.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

