Diagnostic Approach to Pulmonary Hypertension in Premature Neonates
- PMID: 28837121
- PMCID: PMC5615265
- DOI: 10.3390/children4090075
Diagnostic Approach to Pulmonary Hypertension in Premature Neonates
Abstract
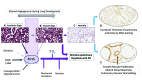
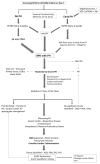
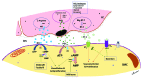
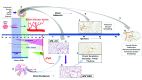
Bronchopulmonary dysplasia (BPD) is a form of chronic lung disease in premature infants following respiratory distress at birth. With increasing survival of extremely low birth weight infants, alveolar simplification is the defining lung characteristic of infants with BPD, and along with pulmonary hypertension, increasingly contributes to both respiratory morbidity and mortality in these infants. Growth restricted infants, infants born to mothers with oligohydramnios or following prolonged preterm rupture of membranes are at particular risk for early onset pulmonary hypertension. Altered vascular and alveolar growth particularly in canalicular and early saccular stages of lung development following mechanical ventilation and oxygen therapy, results in developmental lung arrest leading to BPD with pulmonary hypertension (PH). Early recognition of PH in infants with risk factors is important for optimal management of these infants. Screening tools for early diagnosis of PH are evolving; however, echocardiography is the mainstay for non-invasive diagnosis of PH in infants. Cardiac computed tomography (CT) and magnetic resonance are being used as imaging modalities, however their role in improving outcomes in these patients is uncertain. Follow-up of infants at risk for PH will help not only in early diagnosis, but also in appropriate management of these infants. Aggressive management of lung disease, avoidance of hypoxemic episodes, and optimal nutrition determine the progression of PH, as epigenetic factors may have significant effects, particularly in growth-restricted infants. Infants with diagnosis of PH are managed with pulmonary vasodilators and those resistant to therapy need to be worked up for the presence of cardio-vascular anomalies. The management of infants and toddlers with PH, especially following premature birth is an emerging field. Nonetheless, combination therapies in a multi-disciplinary setting improves outcomes for these infants.
Keywords: bronchopulmonary dysplasia; nitric oxide; premature newborns; pulmonary hypertension; sildenafil.
Conflict of interest statement
The author declares no conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

