Microenvironment inflammatory infiltrate drives growth speed and outcome of hepatocellular carcinoma: a prospective clinical study
- PMID: 28837142
- PMCID: PMC5596578
- DOI: 10.1038/cddis.2017.395
Microenvironment inflammatory infiltrate drives growth speed and outcome of hepatocellular carcinoma: a prospective clinical study
Abstract
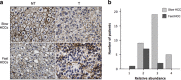
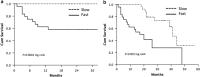
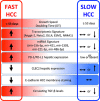
In HCC, tumor microenvironment, heavily influenced by the underlying chronic liver disease, etiology and stage of the tissue damage, affects tumor progression and determines the high heterogeneity of the tumor. Aim of this study was to identify the circulating and tissue components of the microenvironment immune-mediated response affecting the aggressiveness and the ensuing clinical outcome. We analyzed the baseline paired HCC and the surrounding tissue biopsies from a prospective cohort of 132 patients at the first diagnosis of HCC for immunolocalization of PD-1/PD-L1, FoxP3, E-cadherin, CLEC2 and for a panel of 82 microRNA associated with regulation of angiogenesis, cell proliferation, cell signaling, immune control and autophagy. Original microarray data were also explored. Serum samples were analyzed for a panel of 19 cytokines. Data were associated with biochemical data, histopathology and survival. Patients with a more aggressive disease and shorter survival, who we named fast-growing accordingly to the tumor doubling time, at presentation had significantly higher AFP levels, TGF-β1 and Cyphra 21-1 levels. Transcriptomic analysis evidenced a significant downregulation of CLEC2 and upregulation of several metalloproteinases. A marked local upregulation of both PD-1 and PD-L1, a concomitant FoxP3-positive lymphocytic infiltrate, a loss of E-cadherin, gain of epithelial-mesenchymal transition (EMT) phenotype and extreme poor differentiation at histology were also present. Upregulated microRNA in fast-growing HCCs are associated with TGF-β signaling, angiogenesis and inflammation. Our data show that fast HCCs are characterized not only by redundant neo-angiogenesis but also by unique features of distinctively immunosuppressed microenvironment, prominent EMT, and clear-cut activation of TGFβ1 signaling in a general background of long-standing and permanent inflammatory state.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Giannelli G, Rani B, Dituri F, Cao Y, Palasciano G. Moving toward personalised therapy in patients with hepatocellular carcinoma: the role of the microenvironment. Gut 2014; 63: 1668–1676. - PubMed
-
- Jeng KS, Chang CF, Jeng WJ, Sheen IS, Jeng CJ. Heterogeneity of hepatocellular carcinoma contributes to cancer progression. Crit Rev Oncol Hematol 2015; 94: 337–347. - PubMed
-
- Villa E, Critelli R, Lei B, Marzocchi G, Marzocchi G, Cammà C et al. Neoangiogenesis-related genes are hallmarks of fast-growing hepatocellular carcinomas and worst survival. Results from a prospective study. Gut 2016; 65: 861–869. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

