Human neutrophil elastase mediates fibrinolysis shutdown through competitive degradation of plasminogen and generation of angiostatin
- PMID: 28837538
- PMCID: PMC5767103
- DOI: 10.1097/TA.0000000000001685
Human neutrophil elastase mediates fibrinolysis shutdown through competitive degradation of plasminogen and generation of angiostatin
Abstract
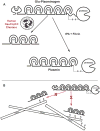
Background: A subset of trauma patients undergo fibrinolysis shutdown rather than pathologic hyperfibrinolysis, contributing to organ failure. The molecular basis for fibrinolysis shutdown in trauma is incompletely understood. Elastase released from primed/activated human neutrophils (HNE) has historically been described as fibrin(ogen)olytic. However, HNE can also degrade plasminogen (PLG) to angiostatin (ANG), retaining the kringle domains but not the proteolytic function, and could thereby compete for generation of active plasmin by tissue plasminogen activator (tPA). We hypothesized that HNE can drive fibrinolysis shutdown rather than fibrinolysis.
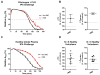
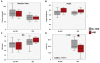
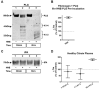
Methods: Turbidometry was performed using light scatter (λ = 620 nm) in a purified fibrinogen + PLG system and in healthy citrate plasma clotted with Ca/thrombin ± tPA, ±HNE, and ±ANG to evaluate HNE effects on fibrinolysis, quantified by time to transition midpoint (Tm). ΔTm from control is reported as percent of control ±95% CI. Purified HNE coincubated with PLG or tPA was analyzed by western blot to identify cleavage products. Exogenous HNE was mixed ex vivo with healthy volunteer blood (n = 7) and used in TEG ± tPA to evaluate effects on fibrinolysis.
Results: HNE did not cause measurable fibrinolysis on fibrin clots, clotted plasma, or whole blood as assessed by turbidometry or TEG in the absence of tPA. Upon tPA treatment, all three methods of evaluating fibrinolysis showed delays and decreases in fibrinolysis caused by HNE relative to control: fibrin clot turbidometry ΔTm = 110.7% (CI 105.0-116.5%), clotted citrate plasma (n = 6 healthy volunteers) ΔTm = 126.1% (CI 110.4-141.8%), and whole blood native TEG (n = 7 healthy volunteers) with ΔLY30 = 28% (p = 0.043). Western blot analysis of HNE-PLG co-incubation confirmed that HNE generates angiostatin K1-3, and plasma turbidity assays treated with angiostatin K1-3 delayed fibrinolysis.
Conclusion: HNE degrades PLG and generates angiostatin K1-3, which predominates over HNE cleavage of fibrin(ogen). These findings suggest that neutrophil release of elastase may underlie trauma-induced fibrinolytic shutdown.
Conflict of interest statement
No conflicts of interest are reported.
Figures

Similar articles
-
Tranexamic acid mediates proinflammatory and anti-inflammatory signaling via complement C5a regulation in a plasminogen activator-dependent manner.J Trauma Acute Care Surg. 2019 Jan;86(1):101-107. doi: 10.1097/TA.0000000000002092. J Trauma Acute Care Surg. 2019. PMID: 30575685
-
Plasma-based assays distinguish hyperfibrinolysis and shutdown subgroups in trauma-induced coagulopathy.J Trauma Acute Care Surg. 2022 Nov 1;93(5):579-587. doi: 10.1097/TA.0000000000003723. Epub 2022 Jun 10. J Trauma Acute Care Surg. 2022. PMID: 35687811 Free PMC article.
-
DNA-bound elastase of neutrophil extracellular traps degrades plasminogen, reduces plasmin formation, and decreases fibrinolysis: proof of concept in septic shock plasma.FASEB J. 2019 Dec;33(12):14270-14280. doi: 10.1096/fj.201901363RRR. Epub 2019 Nov 4. FASEB J. 2019. PMID: 31682515
-
Physiology of plasminogen: with special reference to activation and degradation.Haemostasis. 1988;18 Suppl 1:25-35. doi: 10.1159/000215834. Haemostasis. 1988. PMID: 3280423 Review.
-
Exploitation of plasmin(ogen) by bacterial pathogens of veterinary significance.Vet Microbiol. 2015 Jul 9;178(1-2):1-13. doi: 10.1016/j.vetmic.2015.04.008. Epub 2015 Apr 17. Vet Microbiol. 2015. PMID: 25937317 Review.
Cited by
-
Point-of-care platelet function tests: relevance to arterial thrombosis and opportunities for improvement.J Thromb Thrombolysis. 2021 Jan;51(1):1-11. doi: 10.1007/s11239-020-02170-z. J Thromb Thrombolysis. 2021. PMID: 32529549 Free PMC article. Review.
-
How to manage coagulopathies in critically ill patients.Intensive Care Med. 2023 Mar;49(3):273-290. doi: 10.1007/s00134-023-06980-6. Epub 2023 Feb 17. Intensive Care Med. 2023. PMID: 36808215 Review.
-
Maintaining the balance: the critical role of plasmin activity in orthopedic surgery injury response.J Thromb Haemost. 2023 Oct;21(10):2653-2665. doi: 10.1016/j.jtha.2023.08.002. Epub 2023 Aug 8. J Thromb Haemost. 2023. PMID: 37558131 Free PMC article. Review.
-
Plasma proteomic profile associated with platelet dysfunction after trauma.J Thromb Haemost. 2021 Jul;19(7):1666-1675. doi: 10.1111/jth.15316. Epub 2021 Apr 18. J Thromb Haemost. 2021. PMID: 33774904 Free PMC article.
-
28-day thawed plasma maintains α2 -antiplasmin levels and inhibits tPA-induced fibrinolysis.Vox Sang. 2021 Feb;116(2):181-189. doi: 10.1111/vox.12997. Epub 2020 Sep 7. Vox Sang. 2021. PMID: 32894784 Free PMC article.
References
-
- Moore HB, Moore EE, Gonzalez E, Chapman MP, Chin TL, Silliman CC, Banerjee A, Sauaia A. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: the spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J Trauma Acute Care Surg. 2014;77(6):811–7. discussion -7. - PMC - PubMed
-
- Chapman MP, Moore EE, Moore HB, Gonzalez E, Gamboni F, Chandler JG, Mitra S, Ghasabyan A, Chin TL, Sauaia A, et al. Overwhelming tPA release, not PAI-1 degradation, is responsible for hyperfibrinolysis in severely injured trauma patients. J Trauma Acute Care Surg. 2016;80(1):16–23. discussion -5. - PMC - PubMed
-
- Cardenas JC, Matijevic N, Baer LA, Holcomb JB, Cotton BA, Wade CE. Elevated tissue plasminogen activator and reduced plasminogen activator inhibitor promote hyperfibrinolysis in trauma patients. Shock. 2014;41(6):514–21. - PubMed
-
- Moore HB, Moore EE, Chapman MP, Gonzalez E, Slaughter AL, Morton AP, D'Alessandro A, Hansen KC, Sauaia A, Banerjee A, et al. Viscoelastic measurements of platelet function, not fibrinogen function, predicts sensitivity to tissue-type plasminogen activator in trauma patients. J Thromb Haemost. 2015;13(10):1878–87. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

