National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13-17 Years - United States, 2016
- PMID: 28837546
- PMCID: PMC5687818
- DOI: 10.15585/mmwr.mm6633a2
National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13-17 Years - United States, 2016
Abstract
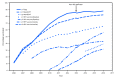
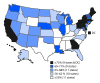
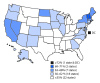
The Advisory Committee on Immunization Practices (ACIP) recommends that adolescents routinely receive tetanus, diphtheria, and acellular pertussis vaccine (Tdap), meningococcal conjugate vaccine (MenACWY), and human papillomavirus (HPV) vaccine (1) at age 11-12 years. ACIP also recommends catch-up vaccination with hepatitis B vaccine, measles, mumps, and rubella (MMR) vaccine, and varicella vaccine for adolescents who are not up to date with childhood vaccinations. ACIP recommends a booster dose of MenACWY at age 16 years (1). In December 2016, ACIP updated HPV vaccine recommendations to include a 2-dose schedule for immunocompetent adolescents initiating the vaccination series before their 15th birthday (2). To estimate adolescent vaccination coverage in the United States, CDC analyzed data from the 2016 National Immunization Survey-Teen (NIS-Teen) for 20,475 adolescents aged 13-17 years.* During 2015-2016, coverage increased for ≥1 dose of Tdap (from 86.4% to 88.0%) and for each HPV vaccine dose (from 56.1% to 60.4% for ≥1 dose). Among adolescents aged 17 years, coverage with ≥2 doses of MenACWY increased from 33.3% to 39.1%. In 2016, 43.4% of adolescents (49.5% of females; 37.5% of males) were up to date with the HPV vaccination series, applying the updated HPV vaccine recommendations retrospectively.† Coverage with ≥1 HPV vaccine dose varied by metropolitan statistical area (MSA) status and was lowest (50.4%) among adolescents living in non-MSA areas and highest (65.9%) among those living in MSA central cities.§ Adolescent vaccination coverage continues to improve overall; however, substantial opportunities exist to further increase HPV-associated cancer prevention.
Conflict of interest statement
Figures
References
-
- Robinson CL, Romero JR, Kempe A, Pellegrini C; Advisory Committee on Immunization Practices (ACIP) Child/Adolescent Immunization Work Group. Advisory Committee on Immunization Practices recommended immunization schedules for persons aged 18 years or younger—United States, 2017. MMWR Morb Mortal Wkly Rep 2017;66:233. 10.15585/mmwr.mm6605e1 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

