Progranulin gene delivery reduces plaque burden and synaptic atrophy in a mouse model of Alzheimer's disease
- PMID: 28837568
- PMCID: PMC5570501
- DOI: 10.1371/journal.pone.0182896
Progranulin gene delivery reduces plaque burden and synaptic atrophy in a mouse model of Alzheimer's disease
Abstract
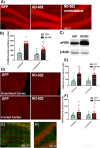
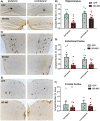
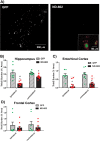
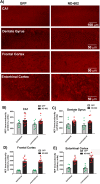
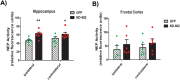
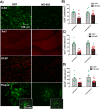
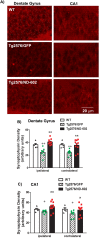
Progranulin (PGRN) is a multifunctional protein that is widely expressed throughout the brain, where it has been shown to act as a critical regulator of CNS inflammation and also functions as an autocrine neuronal growth factor, important for long-term neuronal survival. PGRN has been shown to activate cell signaling pathways regulating excitoxicity, oxidative stress, and synaptogenesis, as well as amyloidogenesis. Together, these critical roles in the CNS suggest that PGRN has the potential to be an important therapeutic target for the treatment of various neurodegenerative disorders, particularly Alzheimer's disease (AD). AD is the leading cause of dementia and is marked by the appearance of extracellular plaques consisting of aggregates of amyloid-β (Aβ), as well as neuroinflammation, oxidative stress, neuronal loss and synaptic atrophy. The ability of PGRN to target multiple key features of AD pathophysiology suggests that enhancing its expression may benefit this disease. Here, we describe the application of PGRN gene transfer using in vivo delivery of lentiviral expression vectors in a transgenic mouse model of AD. Viral vector delivery of the PGRN gene effectively enhanced PGRN expression in the hippocampus of Tg2576 mice. This elevated PGRN expression significantly reduced amyloid plaque burden in these mice, accompanied by reductions in markers of inflammation and synaptic atrophy. The overexpression of PGRN was also found to increase activity of neprilysin, a key amyloid beta degrading enzyme. PGRN regulation of neprilysin activity could play a major role in the observed alterations in plaque burden. Thus, PGRN may be an effective therapeutic target for the treatment of AD.
Conflict of interest statement
Figures
References
-
- Baker M, MacKenzie IR, Pickering-Brown SM, J. G, Rademakers R, Lindholm C, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–9. doi: 10.1038/nature05016 - DOI - PubMed
-
- Ahmed Z, MacKenzie IR, Hutton M, Dickson D. Progranulin in frontotemporal lobar degeneration and neuroinflammation. J Neuroinflammation. 2007;4:7 doi: 10.1186/1742-2094-4-7 - DOI - PMC - PubMed
-
- Ohmi K, Greenberg DS, Rajavel KS, Ryazantsev S, Li HH, Neufeld EF. Activated microglia in cortex of mouse models of mucopolysaccharidoses I and IIIB. Proc Natl Acad Sci U S A. 2003;100(4):1902–7. doi: 10.1073/pnas.252784899 - DOI - PMC - PubMed
-
- Irwin D, Lippa CF, Rosso A. Progranulin (PGRN) expression in ALS: an immunohistochemical study. Journal of Neurological Science. 2009;276(1–2):9–13. - PubMed
-
- Ahmed Z, Sheng H, Xu Y, Lin WL, Innes AE, Gass J, et al. Accelerated lipofuscinosis and ubiquitination in granulin knockout mice suggest a role for progranulin in successful aging. Am J Pathol. 2010;177(1):311–24. doi: 10.2353/ajpath.2010.090915 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

