A two dimensional electromechanical model of a cardiomyocyte to assess intra-cellular regional mechanical heterogeneities
- PMID: 28837585
- PMCID: PMC5570434
- DOI: 10.1371/journal.pone.0182915
A two dimensional electromechanical model of a cardiomyocyte to assess intra-cellular regional mechanical heterogeneities
Abstract
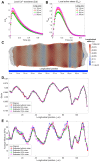
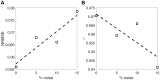
Experimental studies on isolated cardiomyocytes from different animal species and human hearts have demonstrated that there are regional differences in the Ca2+ release, Ca2+ decay and sarcomere deformation. Local deformation heterogeneities can occur due to a combination of factors: regional/local differences in Ca2+ release and/or re-uptake, intra-cellular material properties, sarcomere proteins and distribution of the intracellular organelles. To investigate the possible causes of these heterogeneities, we developed a two-dimensional finite-element electromechanical model of a cardiomyocyte that takes into account the experimentally measured local deformation and cytosolic [Ca2+] to locally define the different variables of the constitutive equations describing the electro/mechanical behaviour of the cell. Then, the model was individualised to three different rat cardiac cells. The local [Ca2+] transients were used to define the [Ca2+]-dependent activation functions. The cell-specific local Young's moduli were estimated by solving an inverse problem, minimizing the error between the measured and simulated local deformations along the longitudinal axis of the cell. We found that heterogeneities in the deformation during contraction were determined mainly by the local elasticity rather than the local amount of Ca2+, while in the relaxation phase deformation was mainly influenced by Ca2+ re-uptake. Our electromechanical model was able to successfully estimate the local elasticity along the longitudinal direction in three different cells. In conclusion, our proposed model seems to be a good approximation to assess the heterogeneous intracellular mechanical properties to help in the understanding of the underlying mechanisms of cardiomyocyte dysfunction.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

