Cost-effectiveness of inactivated seasonal influenza vaccination in a cohort of Thai children ≤60 months of age
- PMID: 28837594
- PMCID: PMC5570265
- DOI: 10.1371/journal.pone.0183391
Cost-effectiveness of inactivated seasonal influenza vaccination in a cohort of Thai children ≤60 months of age
Abstract
Background: Vaccination is the best measure to prevent influenza. We conducted a cost-effectiveness evaluation of trivalent inactivated seasonal influenza vaccination, compared to no vaccination, in children ≤60 months of age participating in a prospective cohort study in Bangkok, Thailand.
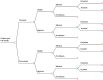
Methods: A static decision tree model was constructed to simulate the population of children in the cohort. Proportions of children with laboratory-confirmed influenza were derived from children followed weekly. The societal perspective and one-year analytic horizon were used for each influenza season; the model was repeated for three influenza seasons (2012-2014). Direct and indirect costs associated with influenza illness were collected and summed. Cost of the trivalent inactivated seasonal influenza vaccine (IIV3) including promotion, administration, and supervision cost was added for children who were vaccinated. Quality-adjusted life years (QALY), derived from literature, were used to quantify health outcomes. The incremental cost-effectiveness ratio (ICER) was calculated as the difference in the expected total costs between the vaccinated and unvaccinated groups divided by the difference in QALYs for both groups.
Results: Compared to no vaccination, IIV3 vaccination among children ≤60 months in our cohort was not cost-effective in the introductory year (2012 season; 24,450 USD/QALY gained), highly cost-effective in the 2013 season (554 USD/QALY gained), and cost-effective in the 2014 season (16,200 USD/QALY gained).
Conclusion: The cost-effectiveness of IIV3 vaccination among children participating in the cohort study varied by influenza season, with vaccine cost and proportion of high-risk children demonstrating the greatest influence in sensitivity analyses. Vaccinating children against influenza can be economically favorable depending on the maturity of the program, influenza vaccine performance, and target population.
Conflict of interest statement
Figures

References
-
- Duncan IG, Taitel MS, Zhang J, Kirkham HS. Planning influenza vaccination programs: a cost benefit model. Cost Eff Resour Alloc. 2012;10(1):10 doi: 10.1186/1478-7547-10-10 - DOI - PMC - PubMed
-
- World Health Organization. Influenza (seasonal) 2016 [cited 2017 April 25]. http://www.who.int/mediacentre/factsheets/fs211/en/.
-
- Hannoun C. The evolving history of influenza viruses and influenza vaccines. Expert Rev Vaccines. 2013;12(9):1085–94. doi: 10.1586/14760584.2013.824709 - DOI - PubMed
-
- Ropero-Alvarez AM, Kurtis HJ, Danovaro-Holliday MC, Ruiz-Matus C, Andrus JK. Expansion of seasonal influenza vaccination in the Americas. BMC Public Health 2009. September 24;9:361 2009;9:361. doi: 10.1186/1471-2458-9-361 - DOI - PMC - PubMed
-
- Hirve S. Seasonal influenza vaccine use in low and middle income countries in tropics and subtropics: a systematic review. Geneva: World Health Organization, 2015.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

