Bispecific T cell engager (BiTE®) antibody constructs can mediate bystander tumor cell killing
- PMID: 28837681
- PMCID: PMC5570333
- DOI: 10.1371/journal.pone.0183390
Bispecific T cell engager (BiTE®) antibody constructs can mediate bystander tumor cell killing
Abstract
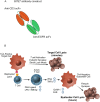
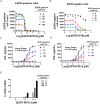
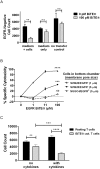
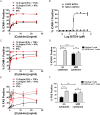
For targets that are homogenously expressed, such as CD19 on cells of the B lymphocyte lineage, immunotherapies can be highly effective. Targeting CD19 with blinatumomab, a CD19/CD3 bispecific antibody construct (BiTE®), or with chimeric antigen receptor T cells (CAR-T) has shown great promise for treating certain CD19-positive hematological malignancies. In contrast, solid tumors with heterogeneous expression of the tumor-associated antigen (TAA) may present a challenge for targeted therapies. To prevent escape of TAA-negative cancer cells, immunotherapies with a local bystander effect would be beneficial. As a model to investigate BiTE®-mediated bystander killing in the solid tumor setting, we used epidermal growth factor receptor (EGFR) as a target. We measured lysis of EGFR-negative populations in vitro and in vivo when co-cultured with EGFR-positive cells, human T cells and an EGFR/CD3 BiTE® antibody construct. Bystander EGFR-negative cells were efficiently lysed by BiTE®-activated T cells only when proximal to EGFR-positive cells. Our mechanistic analysis suggests that cytokines released by BiTE®-activated T-cells induced upregulation of ICAM-1 and FAS on EGFR-negative bystander cells, contributing to T cell-induced bystander cell lysis.
Conflict of interest statement
Figures



References
-
- Baeuerle P, Reinhardt C. Bispecific T-cell engaging antibodies for cancer therapy. Cancer Research. 2009;69(12):4941–4. doi: 10.1158/0008-5472.CAN-09-0547 - DOI - PubMed
-
- Huehls A, Coupet T, Sentman C. Bispecific T-cell engagers for cancer immunotherapy. Immunology and cell biology. 2015;93(3):290–6. doi: 10.1038/icb.2014.93 . - DOI - PMC - PubMed
-
- Klinger M, Benjamin J, Kischel R, Stienen S, Zugmaier G. Harnessing T cells to fight cancer with BiTE antibody constructs—past developments and future directions. Immunological reviews. 2016;270:193–208. doi: 10.1111/imr.12393 - DOI - PubMed
-
- Goebeler M-E, Knop S, Viardot A, Kufer P, Topp MS, Einsele H, et al. Bispecific T-Cell Engager (BiTE) Antibody Construct Blinatumomab for the Treatment of Patients With Relapsed/Refractory Non-Hodgkin Lymphoma: Final Results From a Phase I Study. Journal of Clinical Oncology. 2016. doi: 10.1200/jco.2014.59.1586 - DOI - PubMed
-
- Frankel S, Baeuerle P. Targeting T cells to tumor cells using bispecific antibodies. Current opinion in chemical biology. 2013;17(3):385–92. doi: 10.1016/j.cbpa.2013.03.029 . - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

