Development of MAST: A Microscopy-Based Antimicrobial Susceptibility Testing Platform
- PMID: 28837780
- PMCID: PMC5744253
- DOI: 10.1177/2472630317727721
Development of MAST: A Microscopy-Based Antimicrobial Susceptibility Testing Platform
Abstract
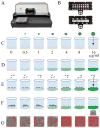
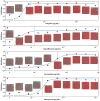
Antibiotic resistance is compromising our ability to treat bacterial infections. Clinical microbiology laboratories guide appropriate treatment through antimicrobial susceptibility testing (AST) of patient bacterial isolates. However, increasingly, pathogens are developing resistance to a broad range of antimicrobials, requiring AST of alternative agents for which no commercially available testing methods are available. Therefore, there exists a significant AST testing gap in which current methodologies cannot adequately address the need for rapid results in the face of unpredictable susceptibility profiles. To address this gap, we developed a multicomponent, microscopy-based AST (MAST) platform capable of AST determinations after only a 2 h incubation. MAST consists of a solid-phase microwell growth surface in a 384-well plate format, inkjet printing-based application of both antimicrobials and bacteria at any desired concentrations, automated microscopic imaging of bacterial replication, and a deep learning approach for automated image classification and determination of antimicrobial minimal inhibitory concentrations (MICs). In evaluating a susceptible strain set, 95.8% were within ±1 and 99.4% were within ±2, twofold dilutions, respectively, of reference broth microdilution MIC values. Most (98.3%) of the results were in categorical agreement. We conclude that MAST offers promise for rapid, accurate, and flexible AST to help address the antimicrobial testing gap.
Keywords: antimicrobials; inkjet printing; machine learning; susceptibility testing.
Figures



References
-
- Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard - tenth edition. CLSI document M07-A10. Clinical and Laboratory Standards Institute; Wayne, PA: 2015.
-
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; twenty-seventh informational supplement. Clinical and Laboratory Standards Institute; Wayne, PA: 2017. CLSI document M100–S27.
-
- Jorgensen JH, Ferraro MJ. Antimicrobial susceptibility testing: a review of general principles and contemporary practices. Clin Infect Dis. 2009;49(11):1749–55. - PubMed
-
- Humphries RM, Hindler JA. Emerging Resistance, New Antimicrobial Agents … but No Tests! The Challenge of Antimicrobial Susceptibility Testing in the Current US Regulatory Landscape. Clin Infect Dis. 2016;63(1):83–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
