Klotho regulates postnatal neurogenesis and protects against age-related spatial memory loss
- PMID: 28837861
- PMCID: PMC5612914
- DOI: 10.1016/j.neurobiolaging.2017.07.008
Klotho regulates postnatal neurogenesis and protects against age-related spatial memory loss
Abstract
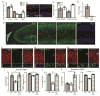
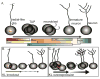
Although the absence of the age-regulating klotho protein causes klotho-deficient mice to rapidly develop cognitive impairment and increasing klotho enhances hippocampal-dependent memory, the cellular effects of klotho that mediate hippocampal-dependent memory function are unknown. Here, we show premature aging of the klotho-deficient hippocampal neurogenic niche as evidenced by reduced numbers of neural stem cells, decreased proliferation, and impaired maturation of immature neurons. Klotho-deficient neurospheres show reduced proliferation and size that is rescued by supplementation with shed klotho protein. Conversely, 6-month-old klotho-overexpressing mice exhibit increased numbers of neural stem cells, increased proliferation, and more immature neurons with enhanced dendritic arborization. Protection from normal age-related loss of object location memory with klotho overexpression and loss of spatial memory when klotho is reduced by even half suggests direct, local effects of the protein. Together, these data show that klotho is a novel regulator of postnatal neurogenesis affecting neural stem cell proliferation and maturation sufficient to impact hippocampal-dependent spatial memory function.
Keywords: Aging; Cognition; Hippocampus; Neural stem cell; Postnatal neurogenesis.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
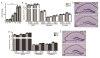



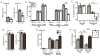
References
-
- Arking DE, Atzmon G, et al. Association between a functional variant of the KLOTHO gene and high-density lipoprotein cholesterol, blood pressure, stroke, and longevity. Circ Res. 2005;96(4):412–418. - PubMed
-
- Ben-Ari Y. Developing networks play a similar melody. Trends in neurosciences. 2001;24(6):353–360. - PubMed
-
- Ben Abdallah NM, Slomianka L, et al. Early age-related changes in adult hippocampal neurogenesis in C57 mice. Neurobiology of aging. 2010;31(1):151–161. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

