The role of infiltrating immune cells in dysfunctional adipose tissue
- PMID: 28838042
- PMCID: PMC5852626
- DOI: 10.1093/cvr/cvx108
The role of infiltrating immune cells in dysfunctional adipose tissue
Abstract
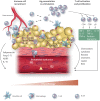
Adipose tissue (AT) dysfunction, characterized by loss of its homeostatic functions, is a hallmark of non-communicable diseases. It is characterized by chronic low-grade inflammation and is observed in obesity, metabolic disorders such as insulin resistance and diabetes. While classically it has been identified by increased cytokine or chemokine expression, such as increased MCP-1, RANTES, IL-6, interferon (IFN) gamma or TNFα, mechanistically, immune cell infiltration is a prominent feature of the dysfunctional AT. These immune cells include M1 and M2 macrophages, effector and memory T cells, IL-10 producing FoxP3+ T regulatory cells, natural killer and NKT cells and granulocytes. Immune composition varies, depending on the stage and the type of pathology. Infiltrating immune cells not only produce cytokines but also metalloproteinases, reactive oxygen species, and chemokines that participate in tissue remodelling, cell signalling, and regulation of immunity. The presence of inflammatory cells in AT affects adjacent tissues and organs. In blood vessels, perivascular AT inflammation leads to vascular remodelling, superoxide production, endothelial dysfunction with loss of nitric oxide (NO) bioavailability, contributing to vascular disease, atherosclerosis, and plaque instability. Dysfunctional AT also releases adipokines such as leptin, resistin, and visfatin that promote metabolic dysfunction, alter systemic homeostasis, sympathetic outflow, glucose handling, and insulin sensitivity. Anti-inflammatory and protective adiponectin is reduced. AT may also serve as an important reservoir and possible site of activation in autoimmune-mediated and inflammatory diseases. Thus, reciprocal regulation between immune cell infiltration and AT dysfunction is a promising future therapeutic target.
Keywords: Adipose tissue; Atherosclerosis; Diabetes; Hypertension; Inflammation.
© The Author. Published on behalf of the European Society of Cardiology.
Figures


References
-
- Norata GD, Caligiuri G, Chavakis T, Matarese G, Netea MG, Nicoletti A, O'neill LA, Marelli-Berg FM.. The cellular and molecular basis of translational immunometabolism. Immunity 2015;43:421–434. - PubMed
-
- Ronti T, Lupattelli G, Mannarino E.. The endocrine function of adipose tissue: an update. Clin Endocrinol (Oxf) 2006;64:355–365. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

