Succeeding in New Vaccine Introduction: Lessons Learned From the Introduction of Inactivated Poliovirus Vaccine in Cameroon, Kenya, and Nigeria
- PMID: 28838156
- PMCID: PMC5853243
- DOI: 10.1093/infdis/jiw544
Succeeding in New Vaccine Introduction: Lessons Learned From the Introduction of Inactivated Poliovirus Vaccine in Cameroon, Kenya, and Nigeria
Abstract
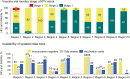
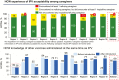
Introducing a new vaccine is a large-scale endeavor that can face many challenges, resulting in introduction delays and inefficiencies. The development of national task teams and tools, such as prelaunch trackers, for the introduction of new vaccines (hereafter, "new vaccine introductions" [NVIs]) can help countries implement robust project management systems, front-load critical preparatory activities, and ensure continuous communication around vaccine supply and financing. In addition, implementing postlaunch assessments to take rapid corrective action accelerates the uptake of the new vaccines. NVIs can provide an opportunity to strengthen routine immunization, through strengthening program management systems or by reinforcing local immunization managers' abilities, among others. This article highlights key lessons learned during the introduction of inactivated poliovirus vaccine in 3 countries that would make future NVIs more successful. The article concludes by considering how the Immunization Systems Management Group of the Global Polio Eradication Initiative has been useful to the NVI process and how such global structures could be further enhanced.
Keywords: IPV; NVI; Polio; RI; program management; technical working groups.
© The Author 2017. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
References
-
- World Health Assembly. Poliomyelitis: Intensification of the global eradication initiative. http://apps.who.int/gb/ebwha/pdf_files/WHA65/A65_R5-en.pdf Accessed 15 August 2016.
-
- Immunization Systems Management Group. Planning for IPV introduction: technical fact sheet, 2013. http://www.who.int/immunization/diseases/poliomyelitis/inactivated_polio... Accessed 15 August 2016.
-
- International Vaccine Access Center, Johns Hopkins Bloomberg School of Public Health. Vaccine Information Management System (VIMS) global vaccine introduction report, 2016. http://www.jhsph.edu/research/centers-and-institutes/ivac/vims/ Accessed 16 August 2016.
-
- Global Polio Eradication Initiative. Background and technical rationale for introduction of one dose of inactivated polio vaccine (IPV) in routine immunization schedule, 2014. http://www.who.int/immunization/diseases/poliomyelitis/inactivated_polio... Accessed 15 April 2016.
-
- World Health Organization. Sage meeting of October 2015: background documents. http://www.who.int/immunization/sage/meetings/2015/october/presentations... Accessed 15 August 2016.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

