Mechanism and Origins of Ligand-Controlled Stereoselectivity of Ni-Catalyzed Suzuki-Miyaura Coupling with Benzylic Esters: A Computational Study
- PMID: 28838241
- PMCID: PMC5607113
- DOI: 10.1021/jacs.7b04973
Mechanism and Origins of Ligand-Controlled Stereoselectivity of Ni-Catalyzed Suzuki-Miyaura Coupling with Benzylic Esters: A Computational Study
Abstract
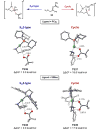
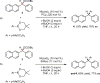
Nickel catalysts have shown unique ligand control of stereoselectivity in the Suzuki-Miyaura cross-coupling of boronates with benzylic pivalates and derivatives involving C(sp3)-O cleavage. The SIMes ligand (1,3-dimesityl-4,5-dihydroimidazol-2-ylidene) produces the stereochemically inverted C-C coupling product, while the tricyclohexylphosphine (PCy3) ligand delivers the retained stereochemistry. We have explored the mechanism and origins of the ligand-controlled stereoselectivity with density functional theory (DFT) calculations. The oxidative addition determines the stereoselectivity with two competing transition states, an SN2 back-side attack type transition state that inverts the benzylic stereogenic center and a concerted oxidative addition through a cyclic transition state, which provides stereoretention. The key difference between the two transition states is the substrate-nickel-ligand angle distortion; the ligand controls the selectivity by differentiating the ease of this angle distortion. For the PCy3 ligand, the nickel-ligand interaction involves mainly σ-donation, which does not require a significant energy penalty for the angle distortion. The facile angle distortion with PCy3 ligand allows the favorable cyclic oxidative addition transition state, leading to the stereoretention. For the SIMes ligand, the extra d-p back-donation from nickel to the coordinating carbene increases the rigidity of the nickel-ligand bond, and the corresponding angle distortion is more difficult. This makes the concerted cyclic oxidative addition unfavorable with SIMes ligand, and the back-side SN2-type oxidative addition delivers the stereoinversion.
Conflict of interest statement
The authors declare no competing financial interest.
Figures












References
-
-
For reviews, see: Yu D-G, Li B-J, Shi Z-J. Acc. Chem. Res. 2010;43:1486.Rosen BM, Quasdorf KW, Wilson DA, Zhang N, Resmerita A-M, Garg NK, Percec V. Chem. Rev. 2011;111:1346.Han F-S. Chem. Soc. Rev. 2013;42:5270.Yamaguchi J, Muto K, Itami K. Eur. J. Org. Chem. 2013;2013:19.Mesganaw T, Garg NK. Org. Process Res. Dev. 2013;17:29.Tasker SZ, Standley EA, Jamison TF. Nature. 2014;509:299.Tobisu M, Chatani N. Top. Curr. Chem. 2016;374:41.Zeng H, Qiu Z, Domínguez-Huerta A, Hearne Z, Chen Z, Li C-J. ACS Catal. 2017;7:510.
-
-
- Shimasaki T, Tobisu M, Chatani N. Angew. Chem. Int. Ed. 2010;49:2929. - PubMed
- Ehle AR, Zhou Q, Watson MP. Org. Lett. 2012;14:1202. - PubMed
- Muto K, Yamaguchi J, Itami K. J. Am. Chem. Soc. 2012;134:169. - PubMed
- Wang J, Ferguson DM, Kalyani D. Tetrahedron. 2013;69:5780.
- Takise R, Muto K, Yamaguchi J, Itami K. Angew. Chem. Int. Ed. 2014;53:6791. - PubMed
- Zarate C, Martin R. J. Am. Chem. Soc. 2014;136:2236. - PubMed
- Koch E, Takise R, Studer A, Yamaguchi J, Itami K. Chem. Commun. 2015;51:855. - PubMed
- Correa A, Leon T, Martin R. J. Am. Chem. Soc. 2014;136:1062. - PubMed
- Kinuta H, Hasegawa J, Tobisu M, Chatani N. Chem. Lett. 2015;44:366.
- Yang J, Chen T, Han L-B. J. Am. Chem. Soc. 2015;137:1782. - PubMed
- Cornella J, Jackson EP, Martin R. Angew. Chem. Int. Ed. 2015;54:4075. - PubMed
- Muto K, Hatakeyama T, Yamaguchi J, Itami K. Chem. Sci. 2015;6:6792. - PMC - PubMed
- Yang J, Xiao J, Chen T, Han L-BJ. J. Org. Chem. 2016;81:3911. - PubMed
- Guo L, Hsiao C-C, Yue H, Liu X, Rueping M. ACS Catal. 2016;6:4438.
- Gu Y, Martin R. Angew. Chem. Int. Ed. 2017;56:3187. - PubMed
- Yue H, Guo L, Liao H-H, Cai Y, Zhu C, Rueping M. Angew. Chem. Int. Ed. 2017;56:4282. - PubMed
- Yue H, Guo L, Liu X, Rueping M. Org. Lett. 2017;19:1788. - PubMed
-
- Sengupta S, Leite M, Raslan DS, Quesnelle C, Snieckus V. J. Org. Chem. 1992;57:4066.
- Dallaire C, Kolber I, Gingras M. Org. Synth. 2002;78:42.
- Quasdorf KW, Riener M, Petrova KV, Garg NK. J. Am. Chem. Soc. 2009;131:17748. - PMC - PubMed
- Antoft-Finch A, Blackburn T, Snieckus V. J. Am. Chem. Soc. 2009;131:17750. - PubMed
- Xi L, Li B-J, Wu Z-H, Lu X-Y, Guan B-T, Wang B-Q, Zhao K-Q, Shi Z-J. Org. Lett. 2010;12:884. - PubMed
- Quasdorf KW, Antoft-Finch A, Liu P, Silberstein AL, Komaromi A, Blackburn T, Ramgren SD, Houk KN, Snieckus V, Garg NK. J. Am. Chem. Soc. 2011;133:6352. - PMC - PubMed
- Mesganaw T, Silberstein AL, Ramgren SD, Nathel NFF, Hong X, Liu P, Garg NK. Chem. Sci. 2011;2:1766. - PMC - PubMed
- Mesganaw T, Nathel NFF, Garg NK. Org. Lett. 2012;14:2918. - PubMed
- Hie L, Ramgren SD, Mesganaw T, Garg NK. Org. Lett. 2012;14:4182. - PMC - PubMed
- Ramgren SD, Hie L, Ye Y-X, Garg NK. Org. Lett. 2013;15:3950. - PMC - PubMed
- Ohtsuki A, Yanagisawa K, Furukawa T, Tobisu M, Chatani N. J. Org. Chem. 2016;81:9409. - PubMed
- Takise R, Itami K, Yamaguchi J. Org. Lett. 2016;18:4428. - PubMed
- Wang Y, Wu SB, Shi WJ, Shi ZJ. Org. Lett. 2016;18:2548. - PubMed
- Shi W-J, Zhao H-W, Wang Y, Cao Z-C, Zhang L-S, Yu D-G, Shi Z-J. Adv. Synth. Catal. 2016;358:2410.
-
- Macklin TK, Snieckus V. Org. Lett. 2005;7:2519. - PubMed
- Wehn PM, Du Bois J. Org. Lett. 2005;7:4685. - PubMed
- Ramgren SD, Silberstein AL, Yang Y, Garg NK. Angew. Chem. Int. Ed. 2011;50:2171. - PMC - PubMed
- Ackermann L, Sandmann R, Song W. Org. Lett. 2011;13:1784. - PubMed
- Chen G-J, Han F-S. Eur. J. Org. Chem. 2012;2012:3575.
- Leowanawat P, Zhang N, Safi M, Hoffman DJ, Fryberger MC, George A, Percec V. J. Org. Chem. 2012;77:2885. - PubMed
- Ke H, Chen X, Zou G. J. Org. Chem. 2014;79:7132. - PubMed
- Park NH, Teverovskiy G, Buchwald SL. Org. Lett. 2014;16:220. - PMC - PubMed
- Mohadjer Beromi M, Nova A, Balcells D, Brasacchio AM, Brudvig GW, Guard LM, Hazari N, Vinyard DJ. J. Am. Chem. Soc. 2017;139:922. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

