Bosutinib versus Placebo for Autosomal Dominant Polycystic Kidney Disease
- PMID: 28838955
- PMCID: PMC5661280
- DOI: 10.1681/ASN.2016111232
Bosutinib versus Placebo for Autosomal Dominant Polycystic Kidney Disease
Abstract
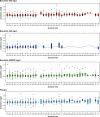
Overactivation of Src has been linked to the pathogenesis of autosomal dominant polycystic kidney disease (ADPKD). This phase 2, multisite study assessed the efficacy and safety of bosutinib, an oral dual Src/Bcr-Abl tyrosine kinase inhibitor, in patients with ADPKD. Patients with ADPKD, eGFR≥60 ml/min per 1.73 m2, and total kidney volume ≥750 ml were randomized 1:1:1 to bosutinib 200 mg/d, bosutinib 400 mg/d, or placebo for ≤24 months. The primary endpoint was annualized rate of kidney enlargement in patients treated for ≥2 weeks who had at least one postbaseline magnetic resonance imaging scan that was preceded by a 30-day washout (modified intent-to-treat population). Of 172 enrolled patients, 169 received at least one study dose. Per protocol amendment, doses for 24 patients who initially received bosutinib at 400 mg/d were later reduced to 200 mg/d. The annual rate of kidney enlargement was reduced by 66% for bosutinib 200 mg/d versus placebo (1.63% versus 4.74%, respectively; P=0.01) and by 82% for pooled bosutinib versus placebo (0.84% versus 4.74%, respectively; P<0.001). Over the treatment period, patients receiving placebo or bosutinib had similar annualized eGFR decline. Gastrointestinal and liver-related adverse events were the most frequent toxicities. In conclusion, compared with placebo, bosutinib at 200 mg/d reduced kidney growth in patients with ADPKD. The overall gastrointestinal and liver toxicity profile was consistent with the profile in prior studies of bosutinib; no new toxicities were identified. (ClinicalTrials.gov: NCT01233869).
Keywords: ADPKD; Src; bosutinib; clinical trial; total kidney volume.
Copyright © 2017 by the American Society of Nephrology.
Figures

References
-
- Torres VE, Harris PC, Pirson Y: Autosomal dominant polycystic kidney disease. Lancet 369: 1287–1301, 2007 - PubMed
-
- Santoro D, Pellicanò V, Visconti L, Trifirò G, Cernaro V, Buemi M: Monoclonal antibodies for renal diseases: Current concepts and ongoing treatments. Expert Opin Biol Ther 15: 1119–1143, 2015 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

