A novel approach to low-temperature synthesis of cubic HfO2 nanostructures and their cytotoxicity
- PMID: 28839143
- PMCID: PMC5570928
- DOI: 10.1038/s41598-017-07753-0
A novel approach to low-temperature synthesis of cubic HfO2 nanostructures and their cytotoxicity
Abstract
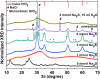
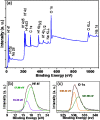
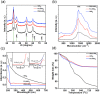
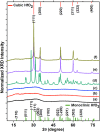
The development of a strategy to stabilise the cubic phase of HfO2 at lower temperatures is necessary for the emergence of unique properties that are not realised in the thermodynamically stable monoclinic phase. A very high temperature (>2600 °C) is required to produce the cubic phase of HfO2, whereas the monoclinic phase is stable at low temperature. Here, a novel rapid synthesis strategy was designed to develop highly crystalline, pure cubic-phase HfO2 nanoparticles (size <10 nm) using microwave irradiation. Furthermore, the as-prepared nanoparticles were converted to different morphologies (spherical nanoparticles and nanoplates) without compromising the cubic phase by employing a post-hydrothermal treatment in the presence of surface modifiers. The cytotoxicities and proliferative profiles of the synthesised cubic HfO2 nanostructures were investigated over the MCF-7 breast cancer cell line, along with caspase-3/7 activities. The low-temperature phase stabilisation was significantly attributed to surface imperfections (defects and deformations) induced in the crystal lattice by the desirable presence of Na2S·xH2O and NaOH. Our work provides unprecedented insight into the stabilisation of nanoscale cubic-phase HfO2 in ambient environments; the method could be extended to other challenging phases of nanomaterials.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Gritsenko VA, Perevalov TV, Islamov DR. Electronic properties of hafnium oxide: A contribution from defects and traps. Phys. Rep. 2016;613:1–20. doi: 10.1016/j.physrep.2015.11.002. - DOI
-
- Matovic B, et al. A novel reduction-oxidation synthetic route for hafnia. Ceram. Int. 2016;42:615–620. doi: 10.1016/j.ceramint.2015.08.155. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

