DACT1 Overexpression in type I ovarian cancer inhibits malignant expansion and cis-platinum resistance by modulating canonical Wnt signalling and autophagy
- PMID: 28839145
- PMCID: PMC5570946
- DOI: 10.1038/s41598-017-08249-7
DACT1 Overexpression in type I ovarian cancer inhibits malignant expansion and cis-platinum resistance by modulating canonical Wnt signalling and autophagy
Erratum in
-
Author Correction: DACT1 Overexpression in type I ovarian cancer inhibits malignant expansion and cis-platinum resistance by modulating canonical Wnt signalling and autophagy.Sci Rep. 2025 Aug 19;15(1):30321. doi: 10.1038/s41598-025-11377-0. Sci Rep. 2025. PMID: 40830376 Free PMC article. No abstract available.
Abstract
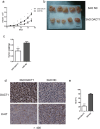
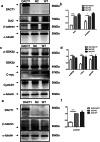
Type I epithelial ovarian cancer (EOC) is primarily resistant to platinum-based chemotherapies and needs novel therapeutics. Given the aberrant Wnt activation in type I EOC and the involvement of Dapper1 Antagonist of Catenin-1 (DACT1) in Wnt signalling, the role of DACT1 in tumourigenesis of type I EOC was evaluated. Firstly, all tested EOC cell lines and primary EOC tissues, especially type I EOC, were observed to have significantly lower DACT1 expression than normal controls. Next, 3AO cells, which arise from a patient with primary mucinous EOC and express low endogenous levels of DACT1, were transfected with a lentivirus carrying full-length DACT1 (3AO-DACT1), grew slower and formed smaller tumours in nude mice compared to 3AO-NC. Furthermore, 3AO-DACT1 had lower levels of key mediators of canonical Wnt signalling, Dvl2 and β-catenin, GSK-3β with phosphorylated Ser9, and the Wnt/β-catenin target genes, with significantly lower nuclear β-catenin levels. Additionally, 3AO-DACT which contained higher levels of lipidated LC3 (LC3-II) and Beclin1, but lower levels of p62/SQSTM1, were more sensitive to cis-platinum. And chloroquine partially rescued its cis-platinum resistance. We identified DACT1 as a negative regulator in type I EOC, protecting against malignant expansion by inhibiting canonical Wnt signalling and cis-platinum resistance by regulating autophagy.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Parmar, M. K. et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet361, 2099–2106 (2003). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

