Closed-loop glucose control in young people with type 1 diabetes during and after unannounced physical activity: a randomised controlled crossover trial
- PMID: 28840263
- PMCID: PMC6448906
- DOI: 10.1007/s00125-017-4395-z
Closed-loop glucose control in young people with type 1 diabetes during and after unannounced physical activity: a randomised controlled crossover trial
Abstract
Aims/hypothesis: Hypoglycaemia during and after exercise remains a challenge. The present study evaluated the safety and efficacy of closed-loop insulin delivery during unannounced (to the closed-loop algorithm) afternoon physical activity and during the following night in young people with type 1 diabetes.
Methods: A randomised, two-arm, open-label, in-hospital, crossover clinical trial was performed at a single site in Slovenia. The order was randomly determined using an automated web-based programme with randomly permuted blocks of four. Allocation assignment was not masked. Children and adolescents with type 1 diabetes who were experienced insulin pump users were eligible for the trial. During four separate in-hospital visits, the participants performed two unannounced exercise protocols: moderate intensity (55% of [Formula: see text]) and moderate intensity with integrated high-intensity sprints (55/80% of [Formula: see text]), using the same study device either for closed-loop or open-loop insulin delivery. We investigated glycaemic control during the exercise period and the following night. The closed-loop insulin delivery was applied from 15:00 h on the day of the exercise to 13:00 h on the following day.
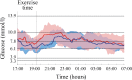
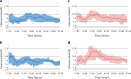
Results: Between 20 January and 16 June 2016, 20 eligible participants (9 female, mean age 14.2 ± 2.0 years, HbA1c 7.7 ± 0.6% [60.0 ± 6.6 mmol/mol]) were included in the trial and performed all trial-mandated activities. The median proportion of time spent in hypoglycaemia below 3.3 mmol/l was 0.00% for both treatment modalities (p = 0.7910). Use of the closed-loop insulin delivery system increased the proportion of time spent within the target glucose range of 3.9-10 mmol/l when compared with open-loop delivery: 84.1% (interquartile range 70.0-85.5) vs 68.7% (59.0-77.7), respectively (p = 0.0057), over the entire study period. This was achieved with significantly less insulin delivered via the closed-loop (p = 0.0123).
Conclusions/interpretation: Closed-loop insulin delivery was safe both during and after unannounced exercise protocols in the in-hospital environment, maintaining glucose values mostly within the target range without an increased risk of hypoglycaemia.
Trial registration: Clinicaltrials.gov NCT02657083 FUNDING: University Medical Centre Ljubljana, Slovenian National Research Agency, and ISPAD Research Fellowship.
Keywords: Clinical science; Devices; Diabetes in childhood; Exercise; Hypoglycaemia.
Conflict of interest statement
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Duality of interest
NB received honoraria for participation on the speaker’s bureau of Medtronic and Roche. RN received honoraria for participation in the speaker’s bureau of Novo Nordisk, Pfizer and Sanofi. RN, TD, OK and TaB own DreaMed stocks. EA and IM are employees of DreaMed Diabetes. OK received honoraria for being on the advisory board of Novo Nordisk as well as speaker’s honoraria from Eli Lilly and Sanofi. ToB received speaker’s honoraria from Medtronic. TD has received speaker’s honoraria and research support from and has consulted for Abbott, AstraZeneca, Bayer, Becton Dickinson, Boehringer, DexCom, Lilly, Medtronic, NovoNordisk, Roche, Sanofi and Ypsomed. MP is a member of the Advisory Board of AstraZeneca, Sanofi, Animas, Medtronic, Bayer Health Care, is a board member of C.G.M.3 Ltd. and is a consultant at Bristol-Myers Squibb, D-medical, Ferring Pharmaceuticals, Andromeda Biotech. The Institute headed by MP received research support from Medtronic, Novo Nordisk, Abbott Diabetes Care, Eli Lilly, Roche, Dexcom, Sanofi, Insulet Corporation, Animas, Andromeda and Macrogenics. MP has been paid lecture fees by Sanofi, Novo Nordisk, Roche and Pfizer. MP is a stock/shareholder of C.G.M.3 Ltd. and DreaMed Diabetes and reports two patent applications. TaB served on advisory boards of Novo Nordisk, Sanofi, Eli Lilly, Boehringer, Medtronic and Bayer Health Care. TaB’s Institution received research grant support, with receipt of travel and accommodation expenses in some cases, from Abbott, Medtronic, Novo Nordisk, GluSense, Sanofi, Sandoz and Diamyd. KD, MM and DL declare that there is no duality of interest associated with their contribution to this manuscript.
Contribution statement
KD, NB, IM, DL, EA, OK, ToB, RN, TD, MP and TaB contributed to the study concept and design. RN, TD, MP and TaB supervised the study. KD, MM, NB and IM collected data. All authors participated in data analysis and interpretation. The manuscript was drafted by KD, MM and TaB, reviewed by KD, MM, DL, EA, OK ToB, RN, TD, MP and TaB and edited by KD and TaB. All contributing authors approved the final version of the manuscript. TaB is the guarantor of the study and takes full responsibility for the work as a whole, including the study design, access to data and the decision to submit and publish the manuscript.
Figures
References
-
- MacMillan F, Kirk A, Mutrie N, Matthews L, Robertson K, Saunders DH. A systematic review of physical activity and sedentary behavior intervention studies in youth with type 1 diabetes: study characteristics, intervention design, and efficacy. Pediatr Diabetes. 2014;15:175–189. doi: 10.1111/pedi.12060. - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

