The enhancement of combination of berberine and metformin in inhibition of DNMT1 gene expression through interplay of SP1 and PDPK1
- PMID: 28840963
- PMCID: PMC5742731
- DOI: 10.1111/jcmm.13347
The enhancement of combination of berberine and metformin in inhibition of DNMT1 gene expression through interplay of SP1 and PDPK1
Abstract
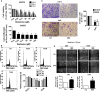
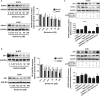
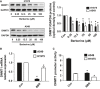
Berberine (BBR), one of active alkaloid found in the rhizome, exhibited anti-cancer properties. We have showed that BBR inhibited growth of non-small cell lung cancer (NSCLC) cells through mitogen-activated protein kinase (MAPK)-mediated increase in forkhead box O3a (FOXO3a). However, the in-depth mechanism underlying the anti-tumor effects still remained to be elucidated. Herein, we further confirmed that BBR not only induced cell cycle arrest, but also reduced migration and invasion of NSCLC cells. Mechanistically, we observed that BBR reduced 3-phosphoinositide-dependent protein kinase-1 (PDPK1) and transcription factor SP1 protein expressions. Exogenously expressed SP1 overcame BBR-inhibited PDPK1 expression. Moreover, BBR inhibited DNA methyltransferase 1 (DNMT1) gene expression and overexpressed DNMT1 resisted BBR-inhibited cell growth. Intriguingly, overexpressed PDPK1 antagonized BBR-inhibited SP1 and DNMT1 expressions. Finally, metformin enhanced the effects of BBR both in vitro and in vivo. Collectively, we observe that BBR inhibits proliferation of NSCLC cells through inhibition of SP1 and PDPK1; this results in a reduction of DNMT1 expression. The interplay of PDPK1 and SP1 contributes to the inhibition of DNMT1 in response to BBR. In addition, there is a synergy of BBR and metformin. This study uncovers a new mechanism of BBR in combination with metformin for NSCLC-associated therapy.
Keywords: Berberine; DNMT1; Metformin; NSCLC; PDPK1; SP1.
© 2017 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures



References
-
- Torre LA, Siegel RL, Jemal A. Lung Cancer Statistics. Adv Exp Med Biol. 2016; 893: 1–19. - PubMed
-
- Dudani S, Leighl NB, Ho C, et al Approach to the non‐operative management of patients with stage II non‐small cell lung cancer (NSCLC): a survey of Canadian medical and radiation oncologists. Lung Cancer. 2016; 94: 74–80. - PubMed
-
- Hussain S. Nanomedicine for Treatment of Lung Cancer. Adv Exp Med Biol. 2016; 890: 137–47. - PubMed
-
- Han Y, Wang H, Xu W, et al Chinese herbal medicine as maintenance therapy for improving the quality of life for advanced non‐small cell lung cancer patients. Complement Ther Med. 2016; 24: 81–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

