Tocilizumab (Actemra)
- PMID: 28841363
- PMCID: PMC5612212
- DOI: 10.1080/21645515.2017.1316909
Tocilizumab (Actemra)
Abstract
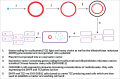
Tocilizumab (TCZ), is a recombinant humanized anti-interleukin-6 receptor (IL-6R) monoclonal antibody which has a main use in the treatment of rheumatoid arthritis, systemic juvenile idiopathic arthritis (sJIA) and polyarticular juvenile idiopathic arthritis (pJIA). This article provides an overview of TCZ including looking into the past at the discovery of interleukin-6 (IL-6) as a pro-inflammatory cytokine. It also looks at how tocilizumab was developed, manufactured and tested to ensure both safety and efficacy in a human population. The article then explores the advantages and disadvantages of using TCZ when compared to other biologics approved in RA, sJIA and pJIA and finally looks ahead to the future and the emerging role of IL-6 and its blockade by TCZ as a treatment for giant cell arteritis (GCA), polymyalgia rheumatica (PMR) and large vessel vasculitis (LVV).
Keywords: giant cell arteritis; interleukin-6; monoclonal antibody; rheumatoid arthritis; tocilizumab; vasculitis.
Figures

References
-
- Assessment Report For RoActemra [Internet] 1st ed. London: European Medicines Agency; 2009. [accessed 2017January3]. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_asses...
-
- RoACTEMRA [Internet] F Hoffmann-La Roche Ltd; c2017 [accessed 2017January2]. http://www.roche.com/products/product-details.htm?productId=30d444d8-765...
-
- ACTEMRA [Internet] Genentech USA; c2016 [accessed 2017January2]. http://www. actemra.com/
-
- Higuchi T, Nakanishi T, Takada K, Matsumoto M, Okada M, Horikoshi H, Suzuki K. A case of multicentric castleman's disease having lung lesion successfully treated with humanized anti-interleukin-6 receptor antibody, Tocilizumab. J Korean Med Sci 2010; 25(9):1364-7; PMID:20808682; https://doi.org/10.3346/jkms.2010.25.9.1364 - DOI - PMC - PubMed
-
- RoActemra 20mg/ml Concentrate for Solution for Infusion Electronic Medicines Compendium; 29/07/16 [Updated 9/August/16; accessed 4/January/17]. Available from: https://www.medicines.org.uk/emc/medicine/22311/SPC/RoActemra+20mg+ml+Co...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
