Efficacy and Safety Outcomes in Patients With Advanced Melanoma Who Discontinued Treatment With Nivolumab and Ipilimumab Because of Adverse Events: A Pooled Analysis of Randomized Phase II and III Trials
- PMID: 28841387
- PMCID: PMC5791828
- DOI: 10.1200/JCO.2017.73.2289
Efficacy and Safety Outcomes in Patients With Advanced Melanoma Who Discontinued Treatment With Nivolumab and Ipilimumab Because of Adverse Events: A Pooled Analysis of Randomized Phase II and III Trials
Abstract
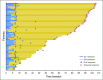
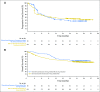
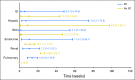
Purpose Approximately 40% of patients with advanced melanoma who received nivolumab combined with ipilimumab in clinical trials discontinued treatment because of adverse events (AEs). We conducted a retrospective analysis to assess the efficacy and safety of nivolumab plus ipilimumab in patients who discontinued treatment because of AEs. Methods Data were pooled from phase II and III trials of patients who received nivolumab 1 mg/kg plus ipilimumab 3 mg/kg, every 3 weeks for four doses, followed by nivolumab monotherapy 3 mg/kg every 2 weeks (N = 409). Efficacy was assessed in all randomly assigned patients who discontinued because of AEs during the induction phase (n = 96) and in those who did not discontinue because of AEs (n = 233). Safety was assessed in treated patients who discontinued because of AEs (n = 176) at any time and in those who did not discontinue because of AEs (n = 231). Results At a minimum follow-up of 18 months, median progression-free survival was 8.4 months for patients who discontinued treatment because of AEs during the induction phase and 10.8 months for patients who did not discontinue because of AEs ( P = .97). Median overall survival had not been reached in either group ( P = .23). The objective response rate was 58.3% for patients who discontinued because of AEs during the induction phase and 50.2% for patients who did not discontinue. The vast majority of grade 3 or 4 AEs occurred during the induction phase, with most resolving after appropriate management. Conclusion Efficacy outcomes seemed similar between patients who discontinued nivolumab plus ipilimumab treatment because of AEs during the induction phase and those who did not discontinue because of AEs. Therefore, even after discontinuation, many patients may continue to derive benefit from combination therapy.
Figures

Comment in
-
Safety and Efficacy Implications of Discontinuing Combination Ipilimumab and Nivolumab in Advanced Melanoma.J Clin Oncol. 2017 Dec 1;35(34):3792-3793. doi: 10.1200/JCO.2017.75.2055. Epub 2017 Oct 19. J Clin Oncol. 2017. PMID: 29048973 No abstract available.
-
Reply to M. Horiguchi et al.J Clin Oncol. 2018 Mar 1;36(7):722-723. doi: 10.1200/JCO.2017.76.5347. Epub 2018 Jan 16. J Clin Oncol. 2018. PMID: 29337635 No abstract available.
-
Patients With Advanced Melanoma Who Discontinued Treatment With Nivolumab and Ipilimumab as a Result of Adverse Events Lived Significantly Longer Than Patients Who Continued Treatment.J Clin Oncol. 2018 Mar 1;36(7):720-721. doi: 10.1200/JCO.2017.76.0983. Epub 2018 Jan 16. J Clin Oncol. 2018. PMID: 29337638 No abstract available.
-
Reply to M. Horiguchi et al.J Clin Oncol. 2018 Mar 1;36(7):721. doi: 10.1200/JCO.2017.76.5339. Epub 2018 Jan 16. J Clin Oncol. 2018. PMID: 29337639 No abstract available.
References
-
- Robert C, Schachter J, Long GV, et al. : Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 372:2521-2532, 2015 - PubMed
-
- Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al: Updated results from a phase III trial of nivolumab (NIVO) combined with ipilimumab (IPI) in treatment-naive patients (pts) with advanced melanoma (MEL) (CheckMate 067). J Clin Oncol 34, 2016 (suppl; abstr 9505)
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

