Infection with purified Piscine orthoreovirus demonstrates a causal relationship with heart and skeletal muscle inflammation in Atlantic salmon
- PMID: 28841684
- PMCID: PMC5571969
- DOI: 10.1371/journal.pone.0183781
Infection with purified Piscine orthoreovirus demonstrates a causal relationship with heart and skeletal muscle inflammation in Atlantic salmon
Abstract
Viral diseases pose a significant threat to the productivity in aquaculture. Heart- and skeletal muscle inflammation (HSMI) is an emerging disease in Atlantic salmon (Salmo salar) farming. HSMI is associated with Piscine orthoreovirus (PRV) infection, but PRV is ubiquitous in farmed Atlantic salmon and thus present also in apparently healthy individuals. This has brought speculations if additional etiological factors are required, and experiments focusing on the causal relationship between PRV and HSMI are highly warranted. A major bottleneck in PRV research has been the lack of cell lines that allow propagation of the virus. To bypass this, we propagated PRV in salmon, bled the fish at the peak of the infection, and purified virus particles from blood cells. Electron microscopy, western blot and high-throughput sequencing all verified the purity of the viral particles. Purified PRV particles were inoculated into naïve Atlantic salmon. The purified virus replicated in inoculated fish, spread to naïve cohabitants, and induced histopathological changes consistent with HSMI. PRV specific staining was demonstrated in the pathological lesions. A dose-dependent response was observed; a high dose of virus gave earlier peak of the viral load and development of histopathological changes compared to a lower dose, but no difference in the severity of the disease. The experiment demonstrated that PRV can be purified from blood cells, and that PRV is the etiological agent of HSMI in Atlantic salmon.
Conflict of interest statement
Figures

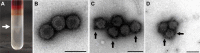


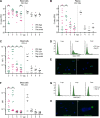
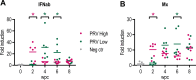




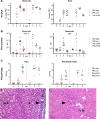
References
-
- Walker PJ, Winton JR. Emerging viral diseases of fish and shrimp. Vet Res. 2010;41(6):51 doi: 10.1051/vetres/2010022 - DOI - PMC - PubMed
-
- Kongtorp RT, Taksdal T, Lyngøy A. Pathology of heart and skeletal muscle inflammation (HSMI) in farmed Atlantic salmon Salmo salar. Dis Aquat Organ. 2004;59(3):217–24. doi: 10.3354/dao059217 - DOI - PubMed
-
- Kongtorp RT, Halse M, Taksdal T, Falk K. Longitudinal study of a natural outbreak of heart and skeletal muscle inflammation in Atlantic salmon, Salmo salar L. J Fish Dis. 2006;29(4):233–44. doi: 10.1111/j.1365-2761.2006.00710.x - DOI - PubMed
-
- Kongtorp RT, Kjerstad A, Taksdal T, Guttvik A, Falk K. Heart and skeletal muscle inflammation in Atlantic salmon, Salmo salar L.: a new infectious disease. J Fish Dis. 2004;27(6):351–8. doi: 10.1111/j.1365-2761.2004.00549.x - DOI - PubMed
-
- Palacios G, Løvoll M, Tengs T, Hornig M, Hutchison S, Hui J, et al. Heart and skeletal muscle inflammation of farmed salmon is associated with infection with a novel reovirus. PLoS One. 2010;5(7):e11487 doi: 10.1371/journal.pone.0011487 - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

