A systematic review of the effectiveness of policies restricting access to pregabalin
- PMID: 28841868
- PMCID: PMC6389065
- DOI: 10.1186/s12913-017-2503-x
A systematic review of the effectiveness of policies restricting access to pregabalin
Abstract
Background: Formularies often employ restriction policies to reduce pharmacy costs. Pregabalin, an alpha-2-delta ligand, is approved for treatment of fibromyalgia (FM); neuropathic pain (NeP) due to postherpetic neuralgia (PHN), diabetic peripheral neuropathy (pDPN), spinal cord injury; and as adjunct therapy for partial onset seizures. Pregabalin is endorsed as first-line therapy for these indications by several US and EU medical professional societies. However, restriction policies such as prior authorization (PA) and step therapy (ST) often favor less costly generic pain medications over pregabalin.
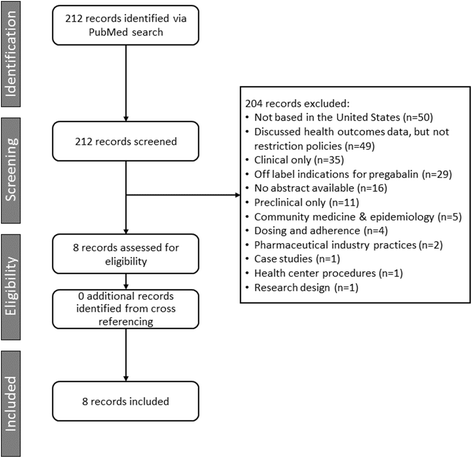
Methods: A structured literature search (PubMed, past 11 years) was conducted to evaluate whether restriction policies against pregabalin support real-world economic and healthcare utilization benefits.
Results: Search criteria identified three claims analyses and a modeling study that evaluated patients with NeP and/or FM with and without PA restrictions; three other studies included patients with FM and NeP in plans with ST requirements, and one evaluated a mail order requirement program. All studies evaluated outcomes during follow-up periods of 6 months or longer. Overall, PA, ST, and mail order restriction policies effectively reduced pregabalin usage, but the effects were inconsistent with reducing pharmacy costs and were non-significant for total disease-related medical costs. Two studies (one PA; one ST) reported significantly higher disease-related costs in restricted plans. The modeling study failed to demonstrate cost savings with PA. Opioid usage was higher in PA-restricted plans (two studies). The US Centers for Disease Control and Prevention and several professional NeP guidelines recommend opioid use only in cases when other non-opioid pain therapies have proven ineffective. New US Government taskforce guidelines now seek to reduce opioid exposure. Additionally, in both ST studies, gabapentin utilization (a common ST edit) was significantly increased. Both studies had substantial proportions of FM and pDPN patients and the only pain condition gabapentin is approved to treat in the United States is PHN.
Conclusion: PA and ST restriction policies significantly decrease utilization of pregabalin, but do not consistently demonstrate cost savings for US health plans. More research is needed to evaluate whether these policies may lead to increased opioid usage as found in some studies.
Trial registration: N/A.
Keywords: Pregabalin; Prior authorization; Restriction policies; Step therapy.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable, as this Research Article was a systematic review and did not enroll patients, but rather, only summarizes previously published literature.
Consent for publication
Not applicable, as no individual patient data are disclosed in this Research Article.
Competing interests
BRS has received current and past support from Pfizer for research unrelated to this review as well as past research support from Teva Pharmaceuticals. JL has worked as a principal investigator on FDA-approved research trials related to pregabalin and has received payment as an advisor and national speaker from many pharmaceutical companies including Pfizer. RB, AS, BP, ETM, and PH are employees and shareholders of Pfizer.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Report Brief. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Institute of Medicine of the National Academies. 2011. http://iom.nationalacademies.org/Reports/2011/Relieving-Pain-in-America-.... Accessed 19 Aug 2015. - PubMed
-
- Epilepsy Fast Facts. Atlanta, GA: Centers for Disease Control and Prevention; 2015. http://www.cdc.gov/epilepsy/basics/fast-facts.htm. Accessed 15 Dec 2015.
-
- Lyrica (pregabalin) [US prescribing information]. New York, NY: Pfizer; 2013. http://labeling.pfizer.com/ShowLabeling.aspx?id=561. Accessed 26 Aug 2015.
-
- Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpää M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice ASC, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. The Lancet Neurology 2015;14(2):162–173. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous