Immediate postpartum levonorgestrel intrauterine device insertion and breast-feeding outcomes: a noninferiority randomized controlled trial
- PMID: 28842126
- PMCID: PMC6040814
- DOI: 10.1016/j.ajog.2017.08.003
Immediate postpartum levonorgestrel intrauterine device insertion and breast-feeding outcomes: a noninferiority randomized controlled trial
Abstract
Background: Immediate postpartum levonorgestrel intrauterine device insertion is increasing in frequency in the United States, but few studies have investigated the effect of early placement on breast-feeding outcomes.
Objective: This study examined the effect of immediate vs delayed postpartum levonorgestrel intrauterine device insertion on breast-feeding outcomes.
Study design: We conducted this noninferiority randomized controlled trial at the University of Utah and the University of New Mexico Health Sciences Centers from February 2014 through March 2016. Eligible women were pregnant and planned to breast-feed, spoke English or Spanish, were aged 18-40 years, and desired a levonorgestrel intrauterine device. Enrolled women were randomized 1:1 to immediate postpartum insertion or delayed insertion at 4-12 weeks' postpartum. Prespecified exclusion criteria included delivery <37.0 weeks' gestational age, chorioamnionitis, postpartum hemorrhage, contraindications to levonorgestrel intrauterine device insertion, and medical complications of pregnancy that could affect breast-feeding. We conducted per-protocol analysis as the primary approach, as it is considered the standard for noninferiority studies; we also report the alternative intent-to-treat analysis. We powered the study for the primary outcome, breast-feeding continuation at 8 weeks, to detect a 15% noninferiority margin between groups, requiring 132 participants in each arm. The secondary study outcome, time to lactogenesis, used a validated measure, and was analyzed by survival analysis and log rank test. We followed up participants for ongoing data collection for 6 months. Only the data analysis team was blinded to the intervention.
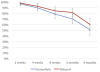
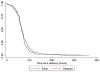
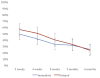
Results: We met the enrollment target with 319 participants, but lost 34 prior to randomization and excluded an additional 26 for medical complications prior to delivery. The final analytic sample included 132 in the immediate group and 127 in the delayed group. Report of any breast-feeding at 8 weeks in the immediate group (79%; 95% confidence interval, 70-86%) was noninferior to that of the delayed group (84%; 95% confidence interval, 76-91%). The 5% difference in breast-feeding continuation at 8 weeks between the groups fell within the noninferiority margin (95% confidence interval, -5.6 to 15%). Time to lactogenesis (mean ± SD) in the immediate group, 65.3 ± 25.7 hours, was noninferior to that of the delayed group, 63.6 ± 21.6 hours. The mean difference between groups was 1.7 hours (95% confidence interval, -4.8 to 8.2 hours), noninferior by log-rank test. A total of 24 intrauterine device expulsions occurred in the immediate group compared to 2 in the delayed group (19% vs 2%, P < .001), consistent with the known higher expulsion rate with immediate vs delayed postpartum intrauterine device insertion. No intrauterine device perforations occurred in either group.
Conclusion: Our results of noninferior breast-feeding outcomes between women with immediate and delayed postpartum levonorgestrel intrauterine device insertion suggest that immediate postpartum intrauterine device insertion is an acceptable option for women planning to breast-feed and use the levonorgestrel intrauterine device. Expulsion rates are higher with immediate postpartum levonorgestrel intrauterine device insertion compared to delayed insertion, but this disadvantage may be outweighed by the advantages of immediate initiation of contraception. Providers should offer immediate postpartum intrauterine device insertion to breast-feeding women planning to use the levonorgestrel intrauterine device.
Keywords: breast-feeding; contraception; immediate postpartum intrauterine device insertion; levonorgestrel intrauterine device.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
References
-
- American Academy of Pediatrics Committee on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics. 2012;129:e827–41. - PubMed
-
- Dykes F, Moran VH. Infant and young child feeding: challenges to implementing a global strategy. Chichester (United Kingdom) and Ames (IA): Wiley-Blackwell; 2009.
-
- Centers for Disease Control and Prevention. [Accessed Oct. 10, 2016];Infant feeding practices study II and its year six follow up. Available at: http://www.cdc.gov/breastfeeding/data/ifps/results.htm.
-
- National Center for Health Statistics. Healthy People 2020 midcourse review. Hyattsville (MD): US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2016.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

