Dedicated Hippocampal Inhibitory Networks for Locomotion and Immobility
- PMID: 28842418
- PMCID: PMC6596740
- DOI: 10.1523/JNEUROSCI.1076-17.2017
Dedicated Hippocampal Inhibitory Networks for Locomotion and Immobility
Abstract
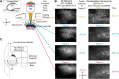
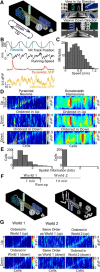
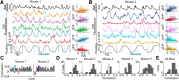
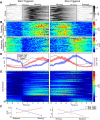
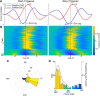
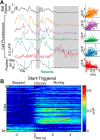
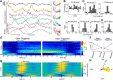
Network activity is strongly tied to animal movement; however, hippocampal circuits selectively engaged during locomotion or immobility remain poorly characterized. Here we examined whether distinct locomotor states are encoded differentially in genetically defined classes of hippocampal interneurons. To characterize the relationship between interneuron activity and movement, we used in vivo, two-photon calcium imaging in CA1 of male and female mice, as animals performed a virtual-reality (VR) track running task. We found that activity in most somatostatin-expressing and parvalbumin-expressing interneurons positively correlated with locomotion. Surprisingly, nearly one in five somatostatin or one in seven parvalbumin interneurons were inhibited during locomotion and activated during periods of immobility. Anatomically, the somata of somatostatin immobility-activated neurons were smaller than those of movement-activated neurons. Furthermore, immobility-activated interneurons were distributed across cell layers, with somatostatin-expressing cells predominantly in stratum oriens and parvalbumin-expressing cells mostly in stratum pyramidale. Importantly, each cell's correlation between activity and movement was stable both over time and across VR environments. Our findings suggest that hippocampal interneuronal microcircuits are preferentially active during either movement or immobility periods. These inhibitory networks may regulate information flow in "labeled lines" within the hippocampus to process information during distinct behavioral states.SIGNIFICANCE STATEMENT The hippocampus is required for learning and memory. Movement controls network activity in the hippocampus but it's unclear how hippocampal neurons encode movement state. We investigated neural circuits active during locomotion and immobility and found interneurons were selectively active during movement or stopped periods, but not both. Each cell's response to locomotion was consistent across time and environments, suggesting there are separate dedicated circuits for processing information during locomotion and immobility. Understanding how the hippocampus switches between different network configurations may lead to therapeutic approaches to hippocampal-dependent dysfunctions, such as Alzheimer's disease or cognitive decline.
Keywords: behavior; calcium imaging; circuits; hippocampus; interneurons; virtual reality.
Copyright © 2017 the authors 0270-6474/17/379222-17$15.00/0.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
