Repulsive Guidance Molecule a (RGMa) Induces Neuropathological and Behavioral Changes That Closely Resemble Parkinson's Disease
- PMID: 28842419
- PMCID: PMC6596765
- DOI: 10.1523/JNEUROSCI.0084-17.2017
Repulsive Guidance Molecule a (RGMa) Induces Neuropathological and Behavioral Changes That Closely Resemble Parkinson's Disease
Abstract
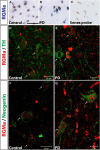
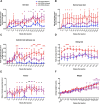
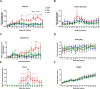
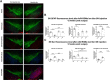
Repulsive guidance molecule member a (RGMa) is a membrane-associated or released guidance molecule that is involved in axon guidance, cell patterning, and cell survival. In our previous work, we showed that RGMa is significantly upregulated in the substantia nigra of patients with Parkinson's disease. Here we demonstrate the expression of RGMa in midbrain human dopaminergic (DA) neurons. To investigate whether RGMa might model aspects of the neuropathology of Parkinson's disease in mouse, we targeted RGMa to adult midbrain dopaminergic neurons using adeno-associated viral vectors. Overexpression of RGMa resulted in a progressive movement disorder, including motor coordination and imbalance, which is typical for a loss of DA release in the striatum. In line with this, RGMa induced selective degeneration of dopaminergic neurons in the substantia nigra (SN) and affected the integrity of the nigrostriatal system. The degeneration of dopaminergic neurons was accompanied by a strong microglia and astrocyte activation. The behavioral, molecular, and anatomical changes induced by RGMa in mice are remarkably similar to the clinical and neuropathological hallmarks of Parkinson's disease. Our data indicate that dysregulation of RGMa plays an important role in the pathology of Parkinson's disease, and antibody-mediated functional interference with RGMa may be a disease modifying treatment option.SIGNIFICANCE STATEMENT Parkinson's disease (PD) is a neurodegenerative disease characterized by severe motor dysfunction due to progressive degeneration of mesencephalic dopaminergic (DA) neurons in the substantia nigra. To date, there is no regenerative treatment available. We previously showed that repulsive guidance molecule member a (RGMa) is upregulated in the substantia nigra of PD patients. Adeno-associated virus-mediated targeting of RGMa to mouse DA neurons showed that overexpression of this repulsive axon guidance and cell patterning cue models the behavioral and neuropathological characteristics of PD in a remarkable way. These findings have implications for therapy development as interfering with the function of this specific axon guidance cue may be beneficial to the survival of DA neurons.
Keywords: AAV-mediated overexpression; Parkinson's disease; RGMa; dopamine neuron degeneration.
Copyright © 2017 the authors 0270-6474/17/379361-19$15.00/0.
Figures






Similar articles
-
Inhibition of repulsive guidance molecule-a protects dopaminergic neurons in a mouse model of Parkinson's disease.Cell Death Dis. 2021 Feb 15;12(2):181. doi: 10.1038/s41419-021-03469-2. Cell Death Dis. 2021. PMID: 33589594 Free PMC article.
-
Cav1.3 channels control D2-autoreceptor responses via NCS-1 in substantia nigra dopamine neurons.Brain. 2014 Aug;137(Pt 8):2287-302. doi: 10.1093/brain/awu131. Epub 2014 Jun 16. Brain. 2014. PMID: 24934288 Free PMC article.
-
Wnt/β-catenin signaling plays an essential role in α7 nicotinic receptor-mediated neuroprotection of dopaminergic neurons in a mouse Parkinson's disease model.Biochem Pharmacol. 2017 Sep 15;140:115-123. doi: 10.1016/j.bcp.2017.05.017. Epub 2017 May 25. Biochem Pharmacol. 2017. PMID: 28551099
-
RGS Proteins as Critical Regulators of Motor Function and Their Implications in Parkinson's Disease.Mol Pharmacol. 2020 Dec;98(6):730-738. doi: 10.1124/mol.119.118836. Epub 2020 Feb 3. Mol Pharmacol. 2020. PMID: 32015009 Free PMC article. Review.
-
Converging roles of ion channels, calcium, metabolic stress, and activity pattern of Substantia nigra dopaminergic neurons in health and Parkinson's disease.J Neurochem. 2016 Oct;139 Suppl 1(Suppl Suppl 1):156-178. doi: 10.1111/jnc.13572. Epub 2016 Mar 23. J Neurochem. 2016. PMID: 26865375 Free PMC article. Review.
Cited by
-
Traumatic brain injury-associated epigenetic changes and the risk for neurodegenerative diseases.Front Neurosci. 2023 Sep 19;17:1259405. doi: 10.3389/fnins.2023.1259405. eCollection 2023. Front Neurosci. 2023. PMID: 37795186 Free PMC article.
-
Update on the Present and Future Pharmacologic Treatment of Parkinson's Disease.Neurol Ther. 2025 Jul 18. doi: 10.1007/s40120-025-00800-3. Online ahead of print. Neurol Ther. 2025. PMID: 40679671 Review.
-
Development and validation of an ultra-performance liquid chromatography with tandem mass spectrometry method for determination of soluble repulsive guidance molecule A in human serum and cerebrospinal fluid.Bioanalysis. 2024;16(21-22):1155-1166. doi: 10.1080/17576180.2024.2403241. Epub 2024 Oct 10. Bioanalysis. 2024. PMID: 39387340 Free PMC article.
-
Repulsive Guidance Molecule-a and Central Nervous System Diseases.Biomed Res Int. 2021 May 4;2021:5532116. doi: 10.1155/2021/5532116. eCollection 2021. Biomed Res Int. 2021. PMID: 33997000 Free PMC article. Review.
-
A primary neural cell culture model to study neuron, astrocyte, and microglia interactions in neuroinflammation.J Neuroinflammation. 2020 May 11;17(1):155. doi: 10.1186/s12974-020-01819-z. J Neuroinflammation. 2020. PMID: 32393376 Free PMC article.
References
-
- Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH, Sherman PM, Holko M, Yefanov A, Lee H, Zhang N, Robertson CL, Serova N, Davis S, Soboleva A (2013) NCBI GEO: archive for functional genomics data sets–update. Nucleic Acids Res 41:D991–D995. - PMC - PubMed
-
- Bossers K, Meerhoff G, Balesar R, van Dongen JW, Kruse CG, Swaab DF, Verhaagen J (2009) Analysis of gene expression in Parkinson's disease: possible involvement of neurotrophic support and axon guidance in dopaminergic cell death. Brain Pathol 19:91–107. 10.1111/j.1750-3639.2008.00171.x - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
