TNF-α promotes nuclear enrichment of the transcription factor TonEBP/NFAT5 to selectively control inflammatory but not osmoregulatory responses in nucleus pulposus cells
- PMID: 28842479
- PMCID: PMC5655530
- DOI: 10.1074/jbc.M117.790378
TNF-α promotes nuclear enrichment of the transcription factor TonEBP/NFAT5 to selectively control inflammatory but not osmoregulatory responses in nucleus pulposus cells
Abstract
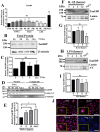
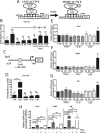
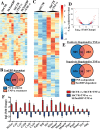
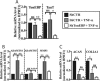
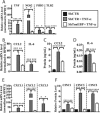
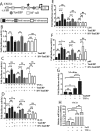
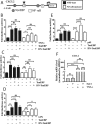
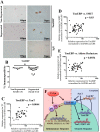
Intervertebral disc degeneration (IDD) causes chronic back pain and is linked to production of proinflammatory molecules by nucleus pulposus (NP) and other disc cells. Activation of tonicity-responsive enhancer-binding protein (TonEBP)/NFAT5 by non-osmotic stimuli, including proinflammatory molecules, occurs in cells involved in immune response. However, whether inflammatory stimuli activate TonEBP in NP cells and whether TonEBP controls inflammation during IDD is unknown. We show that TNF-α, but not IL-1β or LPS, promoted nuclear enrichment of TonEBP protein. However, TNF-α-mediated activation of TonEBP did not cause induction of osmoregulatory genes. RNA sequencing showed that 8.5% of TNF-α transcriptional responses were TonEBP-dependent and identified genes regulated by both TNF-α and TonEBP. These genes were over-enriched in pathways and diseases related to inflammatory response and inhibition of matrix metalloproteases. Based on RNA-sequencing results, we further investigated regulation of novel TonEBP targets CXCL1, CXCL2, and CXCL3 TonEBP acted synergistically with TNF-α and LPS to induce CXCL1-proximal promoter activity. Interestingly, this regulation required a highly conserved NF-κB-binding site but not a predicted TonE, suggesting cross-talk between these two members of the Rel family. Finally, analysis of human NP tissue showed that TonEBP expression correlated with canonical osmoregulatory targets TauT/SLC6A6, SMIT/SLC5A3, and AR/AKR1B1, supporting in vitro findings that the inflammatory milieu during IDD does not interfere with TonEBP osmoregulation. In summary, whereas TonEBP participates in the proinflammatory response to TNF-α, therapeutic strategies targeting this transcription factor for treatment of disc disease must spare osmoprotective, prosurvival, and matrix homeostatic activities.
Keywords: NF-κB (NF-KB); NFAT transcription factor; NFAT5; TonEBP; chemokine; cytokine; inflammation; intervertebral disc; nucleus pulposus.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
References
-
- Burg M. B., Ferraris J. D., and Dmitrieva N. I. (2007) Cellular response to hyperosmotic stresses. Physiol. Rev. 87, 1441–1474 - PubMed
-
- Adams M. A., and Hutton W. C. (1983) The effect of posture on the fluid content of lumbar intervertebral discs. Spine 8, 665–671 - PubMed
-
- Nazari J., Pope M. H., and Graveling R. A. (2015) Feasibility of magnetic resonance imaging (MRI) in obtaining nucleus pulposus (NP) water content with changing postures. Magn. Reson. Imaging 33, 459–464 - PubMed
-
- Tsai T. T., Danielson K. G., Guttapalli A., Oguz E., Albert T. J., Shapiro I. M., and Risbud M. V (2006) TonEBP/OREBP is a regulator of nucleus pulposus cell function and survival in the intervertebral disc. J. Biol. Chem. 281, 25416–25424 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

