The glycosyltransferase GnT-III activates Notch signaling and drives stem cell expansion to promote the growth and invasion of ovarian cancer
- PMID: 28842505
- PMCID: PMC5625063
- DOI: 10.1074/jbc.M117.783936
The glycosyltransferase GnT-III activates Notch signaling and drives stem cell expansion to promote the growth and invasion of ovarian cancer
Abstract
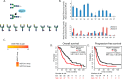
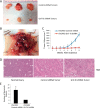
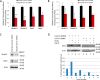
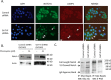
Glycosylation changes associated with cellular transformation can facilitate the growth and progression of tumors. Previously we discovered that the gene Mgat3 encoding the glycosyltransferase GnT-III is elevated in epithelial ovarian carcinomas (EOCs) and leads to the production of abnormal truncated N-linked glycan structures instead of the typical bisected forms. In this study, we are interested in discovering how these abnormal glycans impact the growth and progression of ovarian cancer. We have discovered using stable shRNA gene suppression that GnT-III expression controls the expansion of side-population cells, also known as cancer stem cells. More specifically, we found that GnT-III expression regulates the levels and activation of the heavily glycosylated Notch receptor involved in normal and malignant development. Suppression of GnT-III in EOC cell lines and primary tumor-derived cells resulted in an inhibition of Notch signaling that was more potent than pharmacologic blockage of Notch activation via γ-secretase inhibition. The inhibition resulted from the redirection of the Notch receptor to the lysosome, a novel mechanism. These findings demonstrate a new role for bisecting glycosylation in the control of Notch transport and demonstrate the therapeutic potential of inhibiting GnT-III as a treatment for controlling EOC growth and recurrence.
Keywords: N-linked glycosylation; Notch pathway; cancer; glycosylation; ovarian cancer.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

References
-
- Siegel R., Ma J., Zou Z., and Jemal A. (2014) Cancer statistics, 2014. CA Cancer J. Clin. 64, 9–29 - PubMed
-
- McCluggage W. G. (2011) Morphological subtypes of ovarian carcinoma: a review with emphasis on new developments and pathogenesis. Pathology 43, 420–432 - PubMed
-
- Narasimhan S. (1982) Control of glycoprotein synthesis. UDP-GlcNAc:glycopeptide β 4-N-acetylglucosaminyltransferase III, an enzyme in hen oviduct which adds GlcNAc in β1–4 linkage to the β-linked mannose of the trimannosyl core of N-glycosyl oligosaccharides. J. Biol. Chem. 257, 10235–10242 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

