An aldo-keto reductase is responsible for Fusarium toxin-degrading activity in a soil Sphingomonas strain
- PMID: 28842569
- PMCID: PMC5573404
- DOI: 10.1038/s41598-017-08799-w
An aldo-keto reductase is responsible for Fusarium toxin-degrading activity in a soil Sphingomonas strain
Abstract
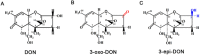
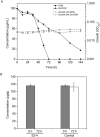
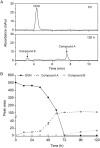
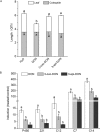
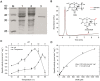
Degradation of toxins by microorganisms is a promising approach for detoxification of agricultural products. Here, a bacterial strain, Sphingomonas S3-4, that has the ability to degrade the mycotoxin deoxynivalenol (DON) was isolated from wheat fields. Incubation of Fusarium-infected wheat grains with S3-4 completely eliminated DON. In S3-4 DON is catabolized into compounds with no detectable phytotoxicity, 3-oxo-DON and 3-epi-DON, via two sequential reactions. Comparative analysis of genome sequences from two DON-degrading strains, S3-4 and Devosia D17, and one non-DON-degrading strain, Sphingobium S26, combined with functional screening of a S3-4 genomic BAC library led to the discovery that a novel aldo/keto reductase superfamily member, AKR18A1, is responsible for oxidation of DON into 3-oxo-DON. DON-degrading activity is completely abolished in a mutant S3-4 strain where the AKR18A1 gene is disrupted. Recombinant AKR18A1 protein expressed in Escherichia coli catalyzed the reversible oxidation/reduction of DON at a wide range of pH values (7.5 to 11) and temperatures (10 to 50 °C). The S3-4 strain and recombinant AKR18A1 also catabolized zearalenone and the aldehydes glyoxal and methyglyoxal. The S3-4 strain and the AKR18A1 gene are promising agents for the control of Fusarium pathogens and detoxification of mycotoxins in plants and in food/feed products.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Zhang JB, et al. Natural occurrence of Fusarium head blight, mycotoxins and mycotoxin-producing isolates of Fusarium in commercial fields of wheat in Hubei. Plant Pathol. 2013;62:92–102. doi: 10.1111/j.1365-3059.2012.02639.x. - DOI
-
- He JW, Zhou T, Young JC, Boland GJ, Scott PM. Chemical and biological transformations for detoxification of trichothecene mycotoxins in human and animal food chains: a review. Trends Food Sci. Technol. 2010;21:67–76. doi: 10.1016/j.tifs.2009.08.002. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

