A short history of RubisCO: the rise and fall (?) of Nature's predominant CO2 fixing enzyme
- PMID: 28843191
- PMCID: PMC7610757
- DOI: 10.1016/j.copbio.2017.07.017
A short history of RubisCO: the rise and fall (?) of Nature's predominant CO2 fixing enzyme
Abstract
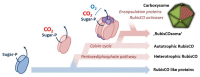
Ribulose-1,5-bisphosphate carboxylase/oxygenase (RubisCO) is arguably one of the most abundant proteins in the biosphere and a key enzyme in the global carbon cycle. Although RubisCO has been intensively studied, its evolutionary origins and rise as Nature's most dominant carbon dioxide (CO2)-fixing enzyme still remain in the dark. In this review we will bring together biochemical, structural, physiological, microbiological, as well as phylogenetic data to speculate on the evolutionary roots of the CO2-fixation reaction of RubisCO, the emergence of RubisCO-based autotrophic CO2-fixation in the context of the Calvin-Benson-Bassham cycle, and the further evolution of RubisCO into the 'RubisCOsome', a complex of various proteins assembling and interacting with the enzyme to improve its operational capacity (functionality) under different biological and environmental conditions.
Copyright © 2017 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures

References
-
- Lorimer GH, Miziorko HM. Carbamate formation on the epsilon-amino group of a lysyl residue as the basis for the activation of ribulosebisphosphate carboxylase by CO2 and Mg2+ . Biochemistry. 1980;19:5321–5328. - PubMed
-
- Anderson LE. Chloroplast and cytoplasmic enzymes. II. Pea leaf triose phosphate isomerases. Biochim Biophys Acta. 1971;235:237–244. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

