The increasing impact of cerebral amyloid angiopathy: essential new insights for clinical practice
- PMID: 28844070
- PMCID: PMC5740546
- DOI: 10.1136/jnnp-2016-314697
The increasing impact of cerebral amyloid angiopathy: essential new insights for clinical practice
Abstract
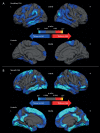
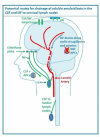
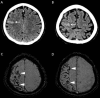
Cerebral amyloid angiopathy (CAA) has never been more relevant. The last 5 years have seen a rapid increase in publications and research in the field, with the development of new biomarkers for the disease, thanks to advances in MRI, amyloid positron emission tomography and cerebrospinal fluid biomarker analysis. The inadvertent development of CAA-like pathology in patients treated with amyloid-beta immunotherapy for Alzheimer's disease has highlighted the importance of establishing how and why CAA develops; without this information, the use of these treatments may be unnecessarily restricted. Our understanding of the clinical and radiological spectrum of CAA has continued to evolve, and there are new insights into the independent impact that CAA has on cognition in the context of ageing and intracerebral haemorrhage, as well as in Alzheimer's and other dementias. While the association between CAA and lobar intracerebral haemorrhage (with its high recurrence risk) is now well recognised, a number of management dilemmas remain, particularly when considering the use of antithrombotics, anticoagulants and statins. The Boston criteria for CAA, in use in one form or another for the last 20 years, are now being reviewed to reflect these new wide-ranging clinical and radiological findings. This review aims to provide a 5-year update on these recent advances, as well as a look towards future directions for CAA research and clinical practice.
Keywords: amyloid; cerebrovascular disease; stroke; superficial siderosis; vascular dementia.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: CC was investigator in clinical trials A9951024 for Pfizer, AstraZeneca and Daiichi-Sankyo, and participated in the scientific boards for Bayer and Medtronic. Fees were paid to ADRINORD or Lille University Hospital research account (no personal funding). SMG serves on safety review committees for immunotherapy trials conducted by Biogen and Hoffman-La Roche. Massachusetts General Hospital participated in the trial of ponezumab under a Clinical Research Support Agreement with Pfizer. DJW was UK chief investigator for A9951024 (Pfizer) and has received consultancy and lecture fees from Bayer.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
