YidC Insertase of Escherichia coli: Water Accessibility and Membrane Shaping
- PMID: 28844594
- PMCID: PMC5675557
- DOI: 10.1016/j.str.2017.07.008
YidC Insertase of Escherichia coli: Water Accessibility and Membrane Shaping
Abstract
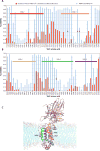
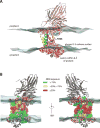
The YidC/Oxa1/Alb3 family of membrane proteins function to insert proteins into membranes in bacteria, mitochondria, and chloroplasts. Recent X-ray structures of YidC from Bacillus halodurans and Escherichia coli revealed a hydrophilic groove that is accessible from the lipid bilayer and the cytoplasm. Here, we explore the water accessibility within the conserved core region of the E. coli YidC using in vivo cysteine alkylation scanning and molecular dynamics (MD) simulations of YidC in POPE/POPG membranes. As expected from the structure, YidC possesses an aqueous membrane cavity localized to the membrane inner leaflet. Both the scanning data and the MD simulations show that the lipid-exposed transmembrane helices 3, 4, and 5 are short, leading to membrane thinning around YidC. Close examination of the MD data reveals previously unrecognized structural features that are likely important for protein stability and function.
Keywords: YidC; alkylation; aqueous access; cysteine-scanning mutagenesis; membrane protein folding; membrane protein insertion; membrane thinning; molecular dynamics.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures





References
-
- Burley SK, Petsko GA. Aromatic-aromatic interaction: A mechanism of protein structure stabilization. Science. 1985;229:23–28. - PubMed
-
- Celebi N, Yi L, Facey SJ, Kuhn A, Dalbey RE. Membrane biogenesis of subunit II of cytochrome bo oxidase: Constrasting requirements for insertion of N-terminal and C-terminal domains. J. Mol. Biol. 2006;357:1428–1436. - PubMed
-
- Chen M, Samuelson JC, Jiang F, Muller M, Kuhn A, Dalbey RE. Direct interaction of YidC with the sec-independent Pf3 coat protein during its membrane protein insertion. J. Biol. Chem. 2002;277:7670–7675. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

