Recapitulation of Human Retinal Development from Human Pluripotent Stem Cells Generates Transplantable Populations of Cone Photoreceptors
- PMID: 28844659
- PMCID: PMC5599247
- DOI: 10.1016/j.stemcr.2017.07.022
Recapitulation of Human Retinal Development from Human Pluripotent Stem Cells Generates Transplantable Populations of Cone Photoreceptors
Abstract
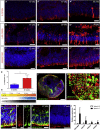
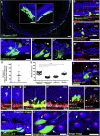
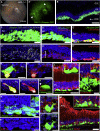
Transplantation of rod photoreceptors, derived either from neonatal retinae or pluripotent stem cells (PSCs), can restore rod-mediated visual function in murine models of inherited blindness. However, humans depend more upon cone photoreceptors that are required for daylight, color, and high-acuity vision. Indeed, macular retinopathies involving loss of cones are leading causes of blindness. An essential step for developing stem cell-based therapies for maculopathies is the ability to generate transplantable human cones from renewable sources. Here, we report a modified 2D/3D protocol for generating hPSC-derived neural retinal vesicles with well-formed ONL-like structures containing cones and rods bearing inner segments and connecting cilia, nascent outer segments, and presynaptic structures. This differentiation system recapitulates human photoreceptor development, allowing the isolation and transplantation of a pure population of stage-matched cones. Purified human long/medium cones survive and become incorporated within the adult mouse retina, supporting the potential of photoreceptor transplantation for treating retinal degeneration.
Keywords: cone photoreceptors; differentiation; photoreceptor; retina and transplantation; stem cells.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

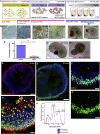
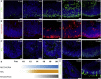
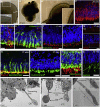
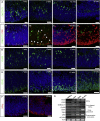
References
-
- Boucherie C., Mukherjee S., Henckaerts E., Thrasher A.J., Sowden J.C., Ali R.R. Brief report: self-organizing neuroepithelium from human pluripotent stem cells facilitates derivation of photoreceptors. Stem Cells. 2013;31:408–414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

