Manumycin A suppresses exosome biogenesis and secretion via targeted inhibition of Ras/Raf/ERK1/2 signaling and hnRNP H1 in castration-resistant prostate cancer cells
- PMID: 28844715
- PMCID: PMC5628151
- DOI: 10.1016/j.canlet.2017.08.020
Manumycin A suppresses exosome biogenesis and secretion via targeted inhibition of Ras/Raf/ERK1/2 signaling and hnRNP H1 in castration-resistant prostate cancer cells
Abstract
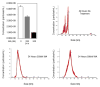
Emerging evidence links exosomes to cancer progression by the trafficking of oncogenic factors and neoplastic reprogramming of stem cells. This necessitates identification and integration of functionally validated exosome-targeting therapeutics into current cancer management regimens. We employed quantitative high throughput screen on two libraries to identify exosome-targeting drugs; a commercially available collection of 1280 pharmacologically active compounds and a collection of 3300 clinically approved compounds. Manumycin-A (MA), a natural microbial metabolite, was identified as an inhibitor of exosome biogenesis and secretion by castration-resistant prostate cancer (CRPC) C4-2B, but not the normal RWPE-1, cells. While no effect was observed on cell growth, MA attenuated ESCRT-0 proteins Hrs, ALIX and Rab27a and exosome biogenesis and secretion by CRPC cells. The MA inhibitory effect is primarily mediated via targeted inhibition of the Ras/Raf/ERK1/2 signaling. The Ras-dependent MA suppression of exosome biogenesis and secretion is partly mediated by ERK-dependent inhibition of the oncogenic splicing factor hnRNP H1. Our findings suggest that MA is a potential drug candidate to suppress exosome biogenesis and secretion by CRPC cells.
Keywords: Exosome biogenesis and secretion; Manumycin A; Prostate cancer; Ras signaling; hnRNP H1.
Copyright © 2017 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

